Enhancing Soil Nitrogen Retention Capacity by Biochar Incorporation in the Acidic Soil of Pomelo Orchards: The Crucial Role of pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Biochar Sampling
2.2. pH Adjustment
2.3. 15N Tracing Experiment
2.4. Laboratory Analyses
2.5. Data and Statistical Analyses
3. Results
3.1. Soil Properties
3.2. NH4+ and NO3− Concentrations and Enrichment during Incubation
3.3. Rates of Gross Transformation of N
3.4. Correlation between Soil Physicochemical Properties and Rates of Gross N Transformation
4. Discussion
4.1. Effects of Biochar Incorporation on TM and TI in Soils at Different pH Values
4.2. Effects of Biochar Incorporation on ONrec and ONH4 in Soils at Different pH Values
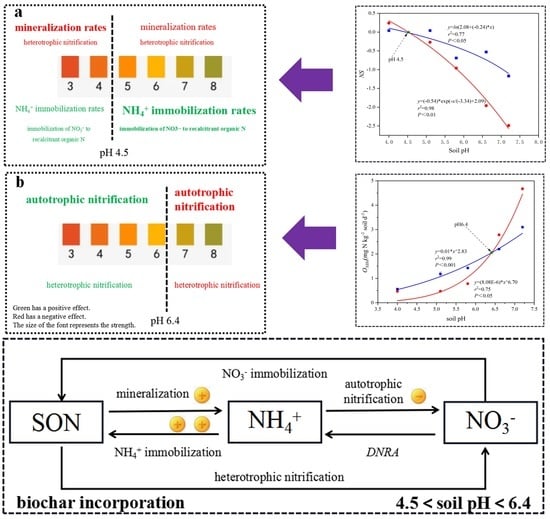
4.3. Nitrogen Retention Mechanism after Biochar Incorporation in Soils at Different pH Values
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.; Feng, Y.; Wang, X.; Yu, Q.; Zhang, F.; Yang, G.; Ren, G.; Han, X.; Wang, X.; Ren, C. Soil Microbial Nitrogen-Cycling Gene Abundances in Response to Crop Diversification: A Meta-Analysis. Sci. Total Environ. 2022, 838, 156621. [Google Scholar] [CrossRef]
- Wang, H.; Liang, J.; Huo, P.; Zhang, L.; Fan, X.; Sun, S. Understanding the Cadmium Passivation and Nitrogen Mineralization of Aminated Lignin in Soil. Sci. Total Environ. 2023, 873, 162334. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Fu, Z.; Zhang, F.; Zhang, R.; Chen, W.; Luo, Y.; Niu, S. Global Patterns and Controlling Factors of Soil Nitrification Rate. Glob. Chang. Biol. 2020, 26, 4147–4157. [Google Scholar] [CrossRef]
- Elrys, A.S.; Chen, Z.; Wang, J.; Uwiragiye, Y.; Helmy, A.M.; Desoky, E.M.; Cheng, Y.; Zhang, J.; Cai, Z.; Müller, C. Global Patterns of Soil Gross Immobilization of Ammonium and Nitrate in Terrestrial Ecosystems. Glob. Chang. Biol. 2022, 28, 4472–4488. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Zheng, X.; Cai, Z.; Misselbrook, T.; Carswell, A.; Müller, C.; Zhang, J. Soil N Transformation Mechanisms Can Effectively Conserve N in Soil under Saturated Conditions Compared to Unsaturated Conditions in Subtropical China. Biol. Fert. Soils 2018, 54, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Liu, Y.; Mo, C.; Jiang, Z.; Yang, J.; Lin, J. Microbial Mechanism of Biochar Addition on Nitrogen Leaching and Retention in Tea Soils from Different Plantation Ages. Sci. Total Environ. 2021, 757, 143817. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Guo, B.; Yu, J.; Carswell, A.; Misselbrook, T.; Zhang, J.; Müller, C.; Chen, D.; Ding, H. Mechanisms behind the Inhibition of Autotrophic Nitrification Following Rice-Straw Incorporation in a Subtropical Acid Soil. Soil Till. Res. 2020, 196, 104436. [Google Scholar] [CrossRef]
- Jiang, Y.; Nyiraneza, J.; Noronha, C.; Mills, A.; Murnaghan, D.; Kostic, A.; Wyand, S. Nitrate Leaching and Potato Tuber Yield Response to Different Crop Rotations. Field Crop. Res. 2022, 288, 108700. [Google Scholar] [CrossRef]
- Wang, S.; Feng, P.; Batchelor, W.D.; Hu, K.; Li, J. Organic Farming Decreases Nitrate Leaching but Increases Dissolved Organic Nitrogen Leaching in Greenhouse Vegetable Production Systems. Plant Soil 2022, 1–14. [Google Scholar] [CrossRef]
- Elrys, A.S.; Ali, A.; Zhang, H.; Cheng, Y.; Zhang, J.; Cai, Z.; Müller, C.; Chang, S.X. Patterns and Drivers of Global Gross Nitrogen Mineralization in Soils. Glob. Chang. Biol. 2021, 27, 5950–5962. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Metwally, M.A.S.; Cheng, Y.; Zhang, J.B.; Cai, Z.; Chang, S.X.; Müller, C. Global Gross Nitrification Rates Are Dominantly Driven by Soil Carbon-to-nitrogen Stoichiometry and Total Nitrogen. Glob. Chang. Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef]
- Cheng, Y.; Elrys, A.S.; Merwad, A.R.M.; Zhang, H.; Chen, Z.; Zhang, J.; Cai, Z.; Müller, C. Global Patterns and Drivers of Soil Dissimilatory Nitrate Reduction to Ammonium. Environ. Sci. Technol. 2022, 56, 3791–3800. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Z. Effects of Land Use Change on Soil Gross Nitrogen Transformation Rates in Subtropical Acid Soils of Southwest China. Environ. Sci. Pollut. Res. 2015, 22, 10850–10860. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, S.; Huang, X.; Zhao, Y.; Zhao, J.; Cheng, Y.; Cai, Z.; Zhang, J. PH-Induced Changes in Fungal Abundance and Composition Affects Soil Heterotrophic Nitrification after 30 Days of Artificial PH Manipulation. Geoderma 2020, 366, 114255. [Google Scholar] [CrossRef]
- Yao, H.; Campbell, C.D.; Qiao, X. Soil PH Controls Nitrification and Carbon Substrate Utilization More than Urea or Charcoal in Some Highly Acidic Soils. Biol. Fert. Soils 2011, 47, 515–522. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Jiang, Y.; Sun, B.; Fan, J.; Zhang, J.; Müller, C.; Cai, Z. Effects of 14 Years of Repeated Pig Manure Application on Gross Nitrogen Transformation in an Upland Red Soil in China. Plant Soil 2017, 415, 161–173. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, X.; Shao, H.; Ma, K. Strong Plant-Soil Associations in a Heterogeneous Subtropical Broad-Leaved Forest. Plant Soil 2011, 347, 211–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhu, T.; Müller, C.; Cai, Z. Effect of Orchard Age on Soil Nitrogen Transformation in Subtropical China and Implications. J. Environ. Sci. 2015, 34, 10–19. [Google Scholar] [CrossRef]
- Chen, X.; Yu, W.; Cai, Y.; Zhang, S.; Muneer, M.A.; Zhu, Q.; Xu, D.; Ma, C.; Yan, X.; Li, Y.; et al. How to Identify and Adopt Cleaner Strategies to Improve the Continuous Acidification in Orchard Soils? J. Clean. Prod. 2022, 330, 129826. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.L.; Zhao, X.R.; Yang, S.H.; Zhang, G.L. Contribution of Different Proton Sources to the Acidification of Red Soil with Maize Cropping in Subtropical China. Geoderma 2021, 392, 114995. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified Soil Acidification from Chemical N Fertilization and Prevention by Manure in an 18-Year Field Experiment in the Red Soil of Southern China. J. Soil. Sediment. 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Y.; Jeyakumar, P.; Lin, L.; Zhang, D.; Yang, M.; Zhu, J.; Ki Lin, C.S.; Wang, H.; Wang, Z.; et al. Soil PH Restricts the Ability of Biochar to Passivate Cadmium: A Meta-Analysis. Environ. Res. 2023, 219, 115110. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Zhang, J.B.; Wang, J.; Cai, Z.C.; Wang, S.Q. Soil PH Is a Good Predictor of the Dominating N2O Production Processes under Aerobic Conditions. J. Plant Nutr. Soil Sci. 2015, 178, 370–373. [Google Scholar] [CrossRef]
- Chen, H.; Hu, W.; Wang, Y.; Zhang, P.; Zhou, Y.; Yang, L.; Li, Y.; Chen, L.; Guo, J. Declined Photosynthetic Nitrogen Use Efficiency under Ammonium Nutrition Is Related to Photosynthetic Electron Transport Chain Disruption in Citrus Plants. Sci. Hortic-Amst. 2023, 308, 111594. [Google Scholar] [CrossRef]
- Teutscherova, N.; Houška, J.; Navas, M.; Masaguer, A.; Benito, M.; Vazquez, E. Leaching of Ammonium and Nitrate from Acrisol and Calcisol Amended with Holm Oak Biochar: A Column Study. Geoderma 2018, 323, 136–145. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum Toxicity in Plants and Its Possible Mitigation in Acid Soils by Biochar: A Review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Masud, M.M.; Baquy, M.A.-A.; Akhter, S.; Sen, R.; Barman, A.; Khatun, M.R. Liming Effects of Poultry Litter Derived Biochar on Soil Acidity Amelioration and Maize Growth. Ecotox. Environ. Safe. 2020, 202, 110865. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, B.; Wu, S.; Feng, H.; Gao, M.; Zhang, B.; Liu, Y. After-Effects of Straw and Straw-Derived Biochar Application on Crop Growth, Yield, and Soil Properties in Wheat (Triticum aestivum L.) -Maize (Zea mays L.) Rotations: A Four-Year Field Experiment. Sci. Total Environ. 2021, 780, 146560. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Rengel, Z.; Du, Z.; Hu, N.; Wang, Y.; Zhang, A. Biochar Application Promotes Crops Yield through Regulating Root Development and the Community Structure of Root Endophytic Fungi in Wheat-Maize Rotation. Soil Till. Res. 2023, 234, 105827. [Google Scholar] [CrossRef]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global Meta-Analysis Reveals Positive Effects of Biochar on Soil Microbial Diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- De La Rosa, J.M.; Rosado, M.; Paneque, M.; Miller, A.Z.; Knicker, H. Effects of Aging under Field Conditions on Biochar Structure and Composition: Implications for Biochar Stability in Soils. Sci. Total Environ. 2018, 613–614, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard Nielsen, H. Effects of Slow and Fast Pyrolysis Biochar on Soil C and N Turnover Dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Aziz, A.A.; Mosharrof, M. Combined Use of Biochar with 15Nitrogen Labelled Urea Increases Rice Yield, N Use Efficiency and Fertilizer N Recovery under Water-Saving Irrigation. Sustainability 2022, 14, 7622. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Tong, C.; Hu, K.; Zhou, B.; Xing, S.; Mao, Y. Biochar-Fertilizer Interaction Modifies N-Sorption, Enzyme Activities and Microbial Functional Abundance Regulating Nitrogen Retention in Rhizosphere Soil. Sci. Total Environ. 2020, 739, 140065. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira Da Silva, J., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Pan, X.; Baquy, M.A.A.; Guan, P.; Yan, J.; Wang, R.; Xu, R.; Xie, L. Effect of Soil Acidification on the Growth and Nitrogen Use Efficiency of Maize in Ultisols. J. Soil. Sediment. 2020, 20, 1435–1445. [Google Scholar] [CrossRef]
- Haque, A.N.A.; Uddin, M.K.; Sulaiman, M.F.; Amin, A.M.; Hossain, M.; Solaiman, Z.M.; Mosharrof, M. Rice Growth Performance, Nutrient Use Efficiency and Changes in Soil Properties Influenced by Biochar under Alternate Wetting and Drying Irrigation. Sustainability 2022, 14, 7977. [Google Scholar] [CrossRef]
- Zheng, J.; Luan, L.; Luo, Y.; Fan, J.; Xu, Q.; Sun, B.; Jiang, Y. Biochar and Lime Amendments Promote Soil Nitrification and Nitrogen Use Efficiency by Differentially Mediating Ammonia-Oxidizer Community in an Acidic Soil. Appl. Soil Ecol. 2022, 180, 104619. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, M.J. Temperature and Substrate Controls on Microbial Phospholipid Fatty Acid Composition during Incubation of Grassland Soils Contrasting in Organic Matter Quality. Soil Biol. Biochem. 2009, 41, 804–812. [Google Scholar] [CrossRef]
- Müller, C.; Rütting, T.; Kattge, J.; Laughlin, R.J.; Stevens, R.J. Estimation of Parameters in Complex 15N Tracing Models by Monte Carlo Sampling. Soil Biol. Biochem. 2007, 39, 715–726. [Google Scholar] [CrossRef]
- Müller, C.; Rütting, T.; Abbasi, M.K.; Laughlin, R.J.; Kammann, C.; Clough, T.J.; Sherlock, R.R.; Kattge, J.; Jäger, H.; Watson, C.J.; et al. Effect of Elevated CO2 on Soil N Dynamics in a Temperate Grassland Soil. Soil Biol. Biochem. 2009, 41, 1996–2001. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Cai, Z.; Müller, C. Nitrogen Cycling in Forest Soils across Climate Gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize Biochars Accelerate Short-Term Soil Nitrogen Dynamics in a Loamy Sand Soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Aciego Pietri, J.C.; Brookes, P.C. Relationships between Soil PH and Microbial Properties in a UK Arable Soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Braos, L.B.; Ruiz, J.G.C.L.; Lopes, I.G.; Ferreira, M.E.; Da Cruz, M.C.P. Mineralization of Nitrogen in Soils with Application of Acid Whey at Different PH. J. Soil Sci. Plant Nut. 2020, 20, 1102–1109. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Pan, J.; Yan, Y.; Niu, S. Chronic Nitrogen Enrichment Decreases Soil Gross Nitrogen Mineralization by Acidification in Topsoil but by Carbon Limitation in Subsoil. Geoderma 2022, 428, 116159. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.; Yang, H.; Zhang, J.; Yang, J.; Cao, J.; Bai, B.; Jiang, Z.; Liang, Y.; et al. Rapid Recovery of Nitrogen Retention Capacity in a Subtropical Acidic Soil Following Afforestation. Soil Biol. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short Term Soil Priming Effects and the Mineralisation of Biochar Following Its Incorporation to Soils of Different PH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Mary, B.; Zhang, J.; Cai, Z.; Chang, S.X. Soil PH Has Contrasting Effects on Gross and Net Nitrogen Mineralizations in Adjacent Forest and Grassland Soils in Central Alberta, Canada. Soil Biol. Biochem. 2013, 57, 848–857. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of Ecosystem Nitrogen Cycle to Nitrogen Addition: A Meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Dai, S.; Meng, L.; Cai, Z.; Zhang, J.; Müller, C. Biochar Application Can Mitigate NH3 Volatilization in Acidic Forest and Upland Soils but Stimulates Gaseous N Losses in Flooded Acidic Paddy Soil. Sci. Total Environ. 2023, 864, 161099. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, C.; Ma, E.; Tan, H.; Zhu, T.; Müller, C. Biochar Stimulates NH4+ Turnover While Decreasing NO3− Production and N2O Emissions in Soils under Long-Term Vegetable Cultivation. Sci. Total Environ. 2020, 737, 140266. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Mao, S.; Chen, Y.; Zhang, J.; Müller, C.; Malghani, S. Combined Effects of Biochar and Biogas Slurry on Soil Nitrogen Transformation Rates and N2O Emission in a Subtropical Poplar Plantation. Sci. Total Environ. 2022, 848, 157766. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, Y.; He, H.; Zhu, T.; Chen, X.; Zhang, X.; Liang, C.; Xie, H.; Zhang, J.; Müller, C.; et al. Effects of Long-Term No-Tillage and Maize Straw Mulching on Gross Nitrogen Transformations in Mollisols of Northeast China. Geoderma 2022, 428, 116194. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, C.; Guo, B.; Yu, J.; Ding, H.; Peng, S.; Zhang, J.; Ireland, E.; Chen, D.; Müller, C.; et al. Mechanisms behind Soil N Dynamics Following Cover Restoration in Degraded Land in Subtropical China. J. Soil. Sediment. 2020, 20, 1897–1905. [Google Scholar] [CrossRef]
- Zhu, T.; Meng, T.; Zhang, J.; Yin, Y.; Cai, Z.; Yang, W.; Zhong, W. Nitrogen Mineralization, Immobilization Turnover, Heterotrophic Nitrification, and Microbial Groups in Acid Forest Soils of Subtropical China. Biol. Fert. Soils 2013, 49, 323–331. [Google Scholar] [CrossRef]
- Zhang, J.; Müller, C.; Cai, Z. Heterotrophic Nitrification of Organic N and Its Contribution to Nitrous Oxide Emissions in Soils. Soil Biol. Biochem. 2015, 84, 199–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Z.; Zhang, J.; Müller, C. The Controlling Factors and the Role of Soil Heterotrophic Nitrification from a Global Review. Appl. Soil Ecol. 2023, 182, 104698. [Google Scholar] [CrossRef]
- Malhi, S.S.; McGill, W.B.; Nyborg, M. Nitrate Losses in Soils: Effect of Temperature, Moisture and Substrate Concentration. Soil Biol. Biochem. 1990, 22, 733–737. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar Additions Alter Phosphorus and Nitrogen Availability in Agricultural Ecosystems: A Meta-Analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of Adding Biochar on Nitrogen Retention and Bioavailability in Agricultural Soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]






| Soil | Biochar | |
|---|---|---|
| pH | 3.7 | 9.3 |
| SOC | 15.3 g kg−1 | 286 g kg−1 |
| TN | 1.3 g kg−1 | 4.6 g kg−1 |
| specific surface area (SSA) | 54.0 |
| Treatment | pH | SOC | TN | DOC | DON |
|---|---|---|---|---|---|
| g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | ||
| S1 | 4.10 i | 14.42 h | 1.61 a | 77.11 bc | 27.07 e |
| S2 | 5.05 g | 16.05 de | 1.77 a | 60.19 f | 27.79 e |
| S3 | 5.65 f | 14.77 gh | 1.63 a | 64.92 ef | 32.93 d |
| S4 | 6.57 c | 17.27 b | 1.88 a | 61.80 f | 33.57 d |
| S5 | 7.03 b | 15.07 fg | 1.65 a | 70.36 cde | 35.34 abc |
| S1 + B | 4.84 h | 15.50 ef | 1.59 a | 50.89 g | 27.85 e |
| S2 + B | 5.82 e | 16.79 bc | 1.71 a | 69.67 de | 34.43 cd |
| S3 + B | 6.37 d | 16.14 d | 1.67 a | 82.60 b | 37.59 abc |
| S4 + B | 7.01 b | 18.46 a | 1.92 a | 89.21 a | 39.43 a |
| S5 + B | 7.56 a | 16.43 cd | 1.73 a | 75.62 cd | 38.95 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Li, Q.; Chen, H.; Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J.; Müller, C.; Yi, Z. Enhancing Soil Nitrogen Retention Capacity by Biochar Incorporation in the Acidic Soil of Pomelo Orchards: The Crucial Role of pH. Agronomy 2023, 13, 2110. https://doi.org/10.3390/agronomy13082110
Qian X, Li Q, Chen H, Zhao L, Wang F, Zhang Y, Zhang J, Müller C, Yi Z. Enhancing Soil Nitrogen Retention Capacity by Biochar Incorporation in the Acidic Soil of Pomelo Orchards: The Crucial Role of pH. Agronomy. 2023; 13(8):2110. https://doi.org/10.3390/agronomy13082110
Chicago/Turabian StyleQian, Xiaojie, Qinghua Li, Hongmei Chen, Lin Zhao, Fei Wang, Yushu Zhang, Jinbo Zhang, Christoph Müller, and Zhigang Yi. 2023. "Enhancing Soil Nitrogen Retention Capacity by Biochar Incorporation in the Acidic Soil of Pomelo Orchards: The Crucial Role of pH" Agronomy 13, no. 8: 2110. https://doi.org/10.3390/agronomy13082110