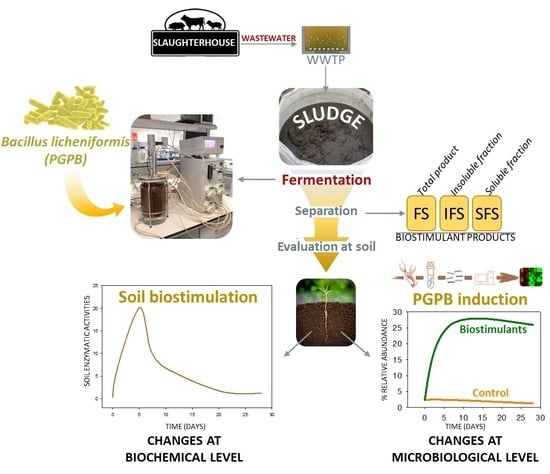
Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus licheniformis-Fermented Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining the Biostimulant
2.2. Chemical Characterization of the Biostimulant Products
2.3. Microbial and Enzymatic Characterization of Fermented Product
2.3.1. B. licheniformis Concentration
2.3.2. Proteomic Study
2.4. Design of the Soil Biostimulation Study
2.5. Soil Analysis
2.5.1. Determination of Soil Enzymatic Activities
2.5.2. Metabarcoding Analysis
2.6. Statistical Analysis
3. Results
3.1. Characterization of Biostimulant Products
3.2. Evaluation of the Biostimulant Capacity of Sludge-Based Products in Soil
3.2.1. Soil Biochemical Properties
3.2.2. Soil Microbiological Characterization
4. Discussion
4.1. Characterization of Biostimulant Products
4.2. Soil Biostimulant Capacity of Biostimulants
4.2.1. Soil Enzymatic Activities
4.2.2. Changes in Soil at Microbiological Level
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, A.M.; Díaz, A.; Tejada, M.; Bautista, J.; Rodríguez, B.; Santa María, C.; Revilla, E.; Parrado, J. Enzymatic production of an organic soil biostimulant from wheat-condensed distiller solubles: Effects on soil biochemistry and biodiversity. Process Biochem. 2010, 45, 1127–1133. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Gómez, I.; Parrado, J.; García-Martínez, A.M.; Aragón, C.; Tejada, M. Obtaining edaphic biostimulants/biofertilizers from different sewage sludges. Effects on soil biological properties. Environ. Technol. 2015, 36, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Caballero, P.; Rodríguez-Morgado, B.; Macías, S.; Tejada, M.; Parrado, J. Obtaining Plant and Soil Biostimulants by Waste Whey Fermentation. Waste Biomass Valorization 2019, 11, 3281–3292. [Google Scholar] [CrossRef]
- Yang, L.; Li, T.; Li, F.; Lemcoff, J.H.; Cohen, S. Fertilization regulates soil enzymatic activity and fertility dynamics in a cucumber field. Sci. Hortic. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Benedicto, S.; Lee, H.C.; Cook, H.F. Enzyme activity and C and N pools in soil following application of mulches. Can. J. Soil Sci. 2004, 84, 19–30. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Parrado, J. Degradation of chlorpyrifos using different biostimulants/biofertilizers: Effects on soil biochemical properties and microbial community. Appl. Soil Ecol. 2014, 84, 158–165. [Google Scholar] [CrossRef]
- Paneque, P.; Caballero, P.; Parrado, J.; Gómez, I.; Tejada, M. Use of a biostimulant obtained from okara in the bioremediation of a soil polluted by used motor car oil. J. Hazard. Mater. 2019, 389, 121820. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrado, J.; Bautista, J.; Romero, E.J.; García-Martínez, A.M.; Friaza, V.; Tejada, M. Production of a carob enzymatic extract: Potential use as a biofertilizer. Bioresour. Technol. 2008, 99, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morgado, B.; Caballero, P.; Paneque, P.; Gómez, I.; Parrado, J.; Tejada, M. Obtaining edaphic biostimulants/biofertilizers from sewage sludge using fermentative processes. Short-time effects on soil biochemical properties. Environ. Technol. 2019, 40, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; García-Martínez, A.M.; Rodríguez-Morgado, B.; Carballo, M.; García-Antras, D.; Aragón, C.; Parrado, J. Obtaining biostimulant products for land application from the sewage sludge of small populations. Ecol. Eng. 2013, 50, 31–36. [Google Scholar] [CrossRef]
- Caballero, P.; Macías-Benítez, S.; Revilla, E.; Tejada, M.; Parrado, J.; Castaño, A. Effect of subtilisin, a protease from Bacillus sp., on soil biochemical parameters and microbial biodiversity. Eur. J. Soil Biol. 2020, 101, 103244. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Michailidou, S.; Pasentsis, K.; Argiriou, A.; Krey, G.; Boziaris, I.S. A meta-barcoding approach to assess and compare the storage temperature-dependent bacterial diversity of gilt-head sea bream (Sparus aurata) originating from fish farms from two geographically distinct areas of Greece. Int. J. Food Microbiol. 2018, 278, 36–43. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1998; ISBN 1873-3336.
- Caballero, P.; Ágabo-García, C.; Solera, R.; Parrado, J.; Pérez, M. Eco-energetic management of activated sludge derived from slaughterhouse wastewater treatment: Pre-treatments for enhancing biogas production under anaerobic conditions. Sustain. Energy Fuels 2020, 4, 5072–5079. [Google Scholar] [CrossRef]
- Parrado, J.; Rodriguez-Morgado, B.; Tejada, M.; Hernandez, T.; Garcia, C. Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme Microb. Technol. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Garcia, C. Anaerobic digestion of straw and piggery wastewaters: II. Optimization of the process. Agrochimica 1994, 38, 195–203. [Google Scholar]
- Macias-Benitez, S.; Garcia-Martinez, A.M.; Caballero Jimenez, P.; Gonzalez, J.M.; Tejada Moral, M.; Parrado Rubio, J. Rhizospheric Organic Acids as Biostimulants: Monitoring Feedbacks on Soil Microorganisms and Biochemical Properties. Front. Plant Sci. 2020, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2013, 2, 321–326. [Google Scholar]
- Voigt, B.; Antelmann, H.; Albrecht, D.; Ehrenreich, A.; Maurer, K.H.; Evers, S.; Gottschalk, G.; Van Dijl, J.M.; Schweder, T.; Hecker, M. Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 2008, 16, 53–68. [Google Scholar] [CrossRef]
- Debosz, K.; Rasmussen, P.H.; Pedersen, A.R. Temporal variations in microbial biomass C and cellulolytic enzyme activity in arable soils: Effects of organic matter input. Appl. Soil Ecol. 1999, 13, 209–218. [Google Scholar] [CrossRef]
- Voigt, B.; Schweder, T.; Sibbald, M.J.J.B.; Albrecht, D.; Ehrenreich, A.; Bernhardt, J.; Feesche, J.; Maurer, K.-H.; Gottschalk, G.; van Dijl, J.M.; et al. The extracellular proteome of Bacillus licheniformis grown in different media and under different nutrient starvation conditions. Proteomics 2006, 6, 268–281. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Overview of Fenton pre-treatment of sludge aiming to enhance anaerobic digestion. Rev. Environ. Sci. Biotechnol. 2015, 14, 453–472. [Google Scholar] [CrossRef]
- Pereira, T.P.; Do Amaral, F.P.; Dall’Asta, P.; Brod, F.C.A.; Arisi, A.C.M. Real-time PCR quantification of the plant growth promoting bacteria Herbaspirillum seropedicae strain SmR1 in maize roots. Mol. Biotechnol. 2014, 56, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Rosconi, F.; Davyt, D.; Martínez, V.; Martínez, M.; Abin-Carriquiry, J.A.; Zane, H.; Butler, A.; de Souza, E.M.; Fabiano, E. Identification and structural characterization of serobactins, a suite of lipopeptide siderophores produced by the grass endophyte Herbaspirillum seropedicae. Environ. Microbiol. 2013, 15, 916–927. [Google Scholar] [CrossRef]
- Estrada, G.A.; Baldani, V.L.D.; de Oliveira, D.M.; Urquiaga, S.; Baldani, J.I. Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 2013, 369, 115–129. [Google Scholar] [CrossRef]
- Das, S.; Sultana, K.W.; Chandra, I. Isolation and Characterization of a Plant Growth-Promoting Bacterium Acinetobacter sp. SuKIC24 From in vitro-Grown Basilicum polystachyon (L.) Moench. Curr. Microbiol. 2021, 78, 2961–2969. [Google Scholar] [CrossRef]
- Chaudhari Bhushan, L.; Chincholkar Sudhir, B.; Rane Makarand, R.; Sarode Prashant, D. Siderophoregenic Acinetobacter calcoaceticus Isolated from Wheat Rhizosphere with Strong PGPR Activity. Malays. J. Microbiol. 2009, 5, 6–12. [Google Scholar]
- Xue, Q.Y.; Chen, Y.; Li, S.M.; Chen, L.F.; Ding, G.C.; Guo, D.W.; Guo, J.H. Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol. Control 2009, 48, 252–258. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, A.; Chen, Y.; Liu, J.; Cao, H. Impacts of silicon addition on arsenic fractionation in soils and arsenic speciation in Panax notoginseng planted in soils contaminated with high levels of arsenic. Ecotoxicol. Environ. Saf. 2018, 162, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.L.; Hu, B.S.; Jiang, Y.H.; Liu, F.Q. Identification and Characterization of Lysobacter enzymogenes as a Biological Control Agent Against Some Fungal Pathogens. Agric. Sci. China 2009, 8, 68–75. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, J.; Chen, J.; Shen, W. Physiological Mechanisms of Improved Smut Resistance in Sugarcane Through Application of Silicon. Front. Plant Sci. 2020, 11, 1587. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, R.; Saravanakumar, D.; Raguchander, T. Combination of endophytic Bacillus and Beauveria for the management of Fusarium wilt and fruit borer in tomato. Pest Manag. Sci. 2014, 70, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhiyan, K.; Keerthana, U.; Nagendran, K.; Prabhukarthikeyan, S.R.; Prabakar, K.; Raguchander, T.; Karthikeyan, G. Multifaceted benefits of Bacillus amyloliquefaciens strain FBZ24 in the management of wilt disease in tomato caused by Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 2018, 103, 92–101. [Google Scholar] [CrossRef]
- Lopes, R.; Tsui, S.; Gonçalves, P.J.R.O.; de Queiroz, M.V. A look into a multifunctional toolbox: Endophytic Bacillus species provide broad and underexploited benefits for plants. World J. Microbiol. Biotechnol. 2018, 34, 94. [Google Scholar] [CrossRef] [PubMed]
- Fasimoye, F.O.; Olajuyigbe, F.M.; Sanni, M.D. Purification and characterization of a thermostable extracellular phytase from Bacillus licheniformis PFBL-03. Prep. Biochem. Biotechnol. 2014, 44, 193–205. [Google Scholar] [CrossRef]
- Sukkasem, P.; Kurniawan, A.; Kao, T.C.; Chuang, H.W. A multifaceted rhizobacterium Bacillus licheniformis functions as a fungal antagonist and a promoter of plant growth and abiotic stress tolerance. Environ. Exp. Bot. 2018, 155, 541–551. [Google Scholar] [CrossRef]
- Castro, J.F.; Nouioui, I.; Asenjo, J.A.; Andrews, B.; Bull, A.T.; Goodfellow, M. New genus-specific primers for PCR identification of Rubrobacter strains. Antonie Leeuwenhoek 2019, 112, 1863–1874. [Google Scholar] [CrossRef] [Green Version]






| Fermented Sludge | Insoluble Fraction of Fermented Sludge | Soluble Fraction of Fermented Sludge | |
|---|---|---|---|
| pH | 8.82 ± 0.09 | 8.82 ± 0.09 | 8.82 ± 0.09 |
| Organic matter % w/w | 71.26 ± 0.31 | 68.88 ± 0.47 | 79.66 ± 0.22 |
| C (% w/w) | 36.20 ± 0.03 | 34.73 ± 1.21 | 40.23 ± 1.07 |
| N (% w/w) | 5.63 ± 0.01 | 4.56 ± 0.40 | 7.94 ± 0.36 |
| P (mg Kg−1) | 17,663.19 ± 0.51 | 18,896.71 ± 0.15 | 6338.29 ± 0.03 |
| K (mg Kg−1) | 4452.18 ± 1.38 | 3521.13 ± 0.79 | 8289.96 ± 1.97 |
| S (mg Kg−1) | 17,219.07 ± 0.42 | 18,853.05 ± 0.15 | 6303.72 ± 0.21 |
| Si (mg Kg−1) | 10,313.57 ± 0.26 | 11,623.00 ± 0.09 | ≤3.72 |
| Sn (mg Kg−1) | ≤0.24 | ≤0.24 | ≤0.24 |
| Al (mg Kg−1) | 5690.70 ± 0.05 | 6570.89 ± 0.04 | 215.99 ± 0.01 |
| Ca (mg Kg−1) | 35,608.16 ± 4.07 | 40,610.33 ± 4.67 | 7955.39 ± 1.84 |
| Cd (mg Kg−1) | ≤0.24 | ≤0.24 | ≤0.24 |
| Cr (mg Kg−1) | 9.06 ± 0.00 | 21.13 ± 0.00 | 2.23 ± 0.00 |
| Cu (mg Kg−1) | 161.13 ± 0.02 | 166.67 ± 0.05 | 134.94 ± 0.04 |
| Fe (mg Kg−1) | 9388.99 ± 0.02 | 10,848.83 ± 0.06 | 1208.18 ± 0.40 |
| Mg (mg Kg−1) | 5014.23 ± 0.02 | 5314.55 ± 0.09 | 2814.13 ± 0.08 |
| Mn (mg Kg−1) | 167.97 ± 0.01 | 192.49 ± 0.01 | 6.69 ± 0.00 |
| Na (mg Kg−1) | 3548.29 ± 0.47 | 2499.06 ± 0.36 | 9209.29 ± 1.57 |
| Ni (mg Kg−1) | 22.57 ± 0.00 | 23.47 ± 0.00 | 7.99 ± 0.00 |
| Pb (mg Kg−1) | 56.96 ± 0.00 | 61.03 ± 0.01 | 30.86 ± 0.00 |
| Zn (mg Kg−1) | 1080.36 ± 0.01 | 1251.17 ± 0.04 | 208.18 ± 0.00 |
| Mo (mg Kg−1) | ≤0.24 | ≤0.24 | ≤0.24 |
| Se (mg Kg−1) | ≤0.47 | ≤0.47 | ≤0.47 |
| Hg (mg Kg−1) | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.01 ± 0.00 |
| Access | Description | Score | Function |
|---|---|---|---|
| Basal proteins of Bacillus in the unfermented sludge | |||
| A0A0M0KXG6 | Chemical-damaging agent resistance protein C | 3.32 | Stress |
| A0A160M9Z6 | Phage tail protein | 2.35 | Structural |
| A0A0K9M8G9 | Formamidase | 3.3 | Hydrolase (Amidase) |
| A0A0J5VPC3 | Peptidase S8 | 5.35 | Hydrolase (Endopeptidase) |
| V6SXF8 | Peptidase S8 | 10.16 | Hydrolase (Endopeptidase) |
| A0A0Q3WA41 | Elongation factor G | 2.71 | Protein synthesis |
| A0A0D6ZBQ0 | Peptide-binding protein | 6.77 | Transport |
| A0A135L4D3 | Peptide ABC transporter substrate-binding protein | 2.17 | Transport |
| A0A160M9B5 | ABC transporter substrate-binding protein | 7.53 | Transport |
| N0ASW2 | Bmp family lipoprotein | 2.14 | Transport |
| Fermented sludge with B. licheniformis | |||
| A0A0M0KXG6 a | Chemical-damaging agent resistance protein C | 3.21 | Stress |
| A0A068NC77 | Cell surface protein | 2.11 | Structural |
| A0A068NDT9 | Collagen adhesion protein | 3.08 | Structural |
| A0A068NE02 | Cell wall anchor domain-containing protein | 3.25 | Structural |
| C3FA89 | Spore coat protein GerQ | 3.04 | Structural |
| F0PM11 | Hydrolase, alpha/beta fold family protein | 3.41 | Hydrolase |
| A0A0C2Y2U4 | Formamidase | 2.55 | Hydrolase (Amidase) |
| A0A0K9M8G9 a | Formamidase | 8.73 | Hydrolase (Amidase) |
| A0A0A8X646 | Aminopeptidase Y (Arg, Lys, Leu preference) | 7.42 | Hydrolase (Aminopeptidase) |
| Q93EJ5 | Leucine aminopeptidase | 4.01 | Hydrolase (Aminopeptidase) |
| T5HJ93 | Aminopeptidase | 4.01 | Hydrolase (Aminopeptidase) |
| W7R6U5 | Aminopeptidase | 4.01 | Hydrolase (Aminopeptidase) |
| A0A0A8XED3 | Subtilisin | 6.68 | Hydrolase (Endopeptidase) |
| A0A0J5VPC3 a | Peptidase S8 | 8.93 | Hydrolase (Endopeptidase) |
| A0A0U1NYI7 | Subtilisin-like serine protease | 2.06 | Hydrolase (Endopeptidase) |
| P29599 | Subtilisin BL | 12.39 | Hydrolase (Endopeptidase) |
| P29600 | Subtilisin Savinase | 5.1 | Hydrolase (Endopeptidase) |
| V6SXF8 a | Peptidase S8 | 11.85 | Hydrolase (Endopeptidase) |
| A0A0D1IL93 | Beta-glucanase | 3.1 | Hydrolase (Glucanase) |
| A0A0W8K3R3 | Beta-glucanase | 3.1 | Hydrolase (Glucanase) |
| D0EWD5 | Beta-1,3-1,4-glucanase | 3.1 | Hydrolase (Glucanase) |
| D7GAY2 | Licheninase | 3.1 | Hydrolase (Glucanase) |
| Q6UNS4 | Beta-1,3-1,4-glucanase | 3.1 | Hydrolase (Glucanase) |
| Q84GK1 | Beta-1,3-1,4-endoglucanase (Fragment) | 3.1 | Hydrolase (Glucanase) |
| Q8GMY0 | Beta-1-3,1-4-endoglucanase | 3.1 | Hydrolase (Glucanase) |
| W7R9E9 | Beta-glucanase | 3.1 | Hydrolase (Glucanase) |
| A0A0K9GB73 | UPF0173 metal-dependent hydrolase AC622_04030 | 3.17 | Hydrolase (Beta-lactamase) |
| A0A068NEN6 | UPF0173 metal-dependent hydrolase BcrFT9_03657 | 2.64 | Hydrolase (Beta-lactamase) |
| A0A164CK25 | Metal-dependent hydrolase (Fragment) | 2.47 | Hydrolase (Beta-lactamase) |
| C3DIS4 | NH(3)-dependent NAD(+) synthetase | 2.37 | Metabolism |
| A0A0C3LQW7 | Uncharacterized protein | 2.62 | Oxidoreductase |
| A0A0Q9HD35 | Uncharacterized protein | 3.53 | Pectin lyase |
| A0A0M2SFX9 | Uncharacterized protein | 2.75 | Protease inhibitor |
| A0A0D1L4A8 | Valine--tRNA ligase | 2.69 | Protein synthesis |
| W7RS29 | Peptide synthetase | 2.82 | Protein synthesis |
| A0A098F6B3 | Phosphatidylethanolamine N-methyltransferase | 1.84 | Transferase |
| A0A072NRD1 | Potassium uptake protein, TrkH family | 2.94 | Transport |
| A0A084GY51 | Peptide-binding protein | 2.78 | Transport |
| A0A098EWU5 | Putative lipoprotein | 3.11 | Transport |
| A0A0A8X5D1 | Oligopeptide ABC transporter, periplasmic oligopeptide-binding protein OppA | 18.95 | Transport |
| A0A0D6ZBQ0 a | Peptide-binding protein | 9.47 | Transport |
| A0A0J1ILE4 | Oligopeptide-binding protein AppA | 2.58 | Transport |
| A0A0J5JQF2 | Peptide ABC transporter substrate-binding protein | 3.44 | Transport |
| A0A0M2SQW2 | Peptide ABC transporter substrate-binding protein OS=Bacillus sp. SA2-6 GN=WQ57_16375 PE=4 SV=1 - [A0A0M2SQW2_9BACI] | 2.36 | Transport |
| A0A0M3RFD5 | Peptide ABC transporter substrate-binding protein | 3.85 | Transport |
| A0A150MCH0 | Uncharacterized protein | 25.07 | Transport |
| E5WE49 | Oligopeptide ABC transporter | 6.38 | Transport |
| Q2B8Z3 | Oligopeptide ABC transporter (Binding protein) | 3.07 | Transport |
| V6T2D8 | Uncharacterized protein | 20.84 | Transport |
| W4RN39 | Oligopeptide ABC transporter | 6.3 | Transport |
| A0A068N9Y0 | Conserved repeat domain protein | 6.34 | Unknown |
| A0A0B5NMV1 | Uncharacterized protein | 4.35 | Unknown |
| A0A0D6Z7I3 | Uncharacterized protein | 5.9 | Unknown |
| A0A164D5Z4 | Putative internalin | 2.62 | Unknown |
| J7WX50 | Uncharacterized protein | 3.32 | Unknown |
| Treatment/Day | Goods_Coverage | Observed_Otus | Shannon | Simpson | PD_Whole_Tree | Chao1 | Dominance | |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 1.000 | 165.667 ± 11.671 | 6.830 ± 0.074 | 0.984 ± 0.001 | 9.465 ± 0.593 | 165.667 ± 11.671 | 0.016 ± 0.001 |
| 5 | 1.000 | 169.333 ± 11.672 | 6.558 ± 0.169 | 0.974 ± 0.002 | 11.166 ± 0.522 | 169.333 ± 11.672 | 0.026 ± 0.002 | |
| 28 | 1.000 | 186.667 ± 28.987 | 6.531 ± 0.228 | 0.966 ± 0.005 | 14.227 ± 0.705 | 186.667 ± 28.987 | 0.034 ± 0.005 | |
| FS | 0 | 1.000 | 152.000 ± 15.578 | 6.272 ± 0.153 | 0.972 ± 0.003 | 9.592 ± 0.476 | 152.000 ± 15.578 | 0.028 ± 0.003 |
| 5 | 1.000 | 163.333 ± 9.286 | 6.823 ± 0.040 | 0.987 ± 0.001 | 9.946 ± 0.339 | 163.333 ± 9.286 | 0.013 ± 0.001 | |
| 28 | 1.000 | 167.000 ± 7.000 | 6.602 ± 0.08 | 0.977 ± 0.000 | 11.982 ± 0.206 | 167.000 ± 7.000 | 0.023 ± 0.000 | |
| IFS | 0 | 1.000 | 165.667 ± 15.326 | 6.301 ± 0.169 | 0.971 ± 0.004 | 9.585 ± 0.060 | 165.667 ± 15.326 | 0.029 ± 0.004 |
| 5 | 1.000 | 139.667 ± 12.658 | 6.619 ± 0.101 | 0.984 ± 0.001 | 9.092 ± 0.469 | 139.667 ± 12.658 | 0.016 ± 0.001 | |
| 28 | 1.000 | 209.333 ± 11.557 | 7.031 ± 0.082 | 0.986 ± 0.001 | 13.850 ± 0.478 | 209.333 ± 11.557 | 0.014 ± 0.001 | |
| SFS | 0 | 1.000 | 175.667 ± 11.671 | 6.922 ± 0.080 | 0.985 ± 0.001 | 11.045 ± 0.330 | 175.667 ± 11.671 | 0.015 ± 0.001 |
| 5 | 1.000 | 148.333 ± 5.793 | 6.435 ± 0.072 | 0.975 ± 0.002 | 7.987 ± 0.119 | 148.333 ± 5.793 | 0.025 ± 0.002 | |
| 28 | 1.000 | 159.333 ± 21.061 | 6.540 ± 0.159 | 0.981 ± 0.002 | 9.866 ± 0.636 | 159.333 ± 21.061 | 0.019 ± 0.002 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, P.; Macías-Benítez, S.; Moya, A.; Rodríguez-Morgado, B.; Martín, L.; Tejada, M.; Castaño, A.; Parrado Rubio, J. Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus licheniformis-Fermented Sludge. Agronomy 2022, 12, 1743. https://doi.org/10.3390/agronomy12081743
Caballero P, Macías-Benítez S, Moya A, Rodríguez-Morgado B, Martín L, Tejada M, Castaño A, Parrado Rubio J. Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus licheniformis-Fermented Sludge. Agronomy. 2022; 12(8):1743. https://doi.org/10.3390/agronomy12081743
Chicago/Turabian StyleCaballero, Pablo, Sandra Macías-Benítez, Ana Moya, Bruno Rodríguez-Morgado, Luis Martín, Manuel Tejada, Angélica Castaño, and Juan Parrado Rubio. 2022. "Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus licheniformis-Fermented Sludge" Agronomy 12, no. 8: 1743. https://doi.org/10.3390/agronomy12081743