Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture
Abstract
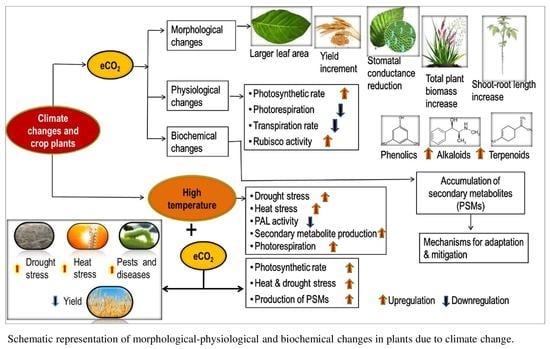
:1. Introduction
2. Impact of Climate Change on Various Physiological and Biochemical Attributes
2.1. Photosynthesis
2.2. Canopy Temperature and Transpiration
2.3. Stability of Membranes
2.4. Photorespiration and Respiration
2.5. Redox Status
2.6. Yield
2.7. Quality
3. Conclusions
4. Further Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A Meta-Analysis. IJERPH 2018, 15, 839. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.B.; Dermody, O.; Klein, S.P.; Locke, A.M.; McGrath, J.M.; Paul, R.E.; Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Strellner, R.; et al. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat. Plants 2016, 2, 16132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhang, X.; Li, L.; Lam, S.K.; Pan, G. Changes in plant C, N and P ratios under elevated [CO2] and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 2019, 9, 5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Turkan, I.; Demiral, T.; Sekmen, A.H. The regulation of antioxidant enzymes in two Plantago species differing in salinity tolerance under combination of waterlogging and salinity. Funct. Plant Biol. 2013, 40, 484. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, D.; Agrawal, S.B. Ultraviolet-B induced changes in physiology, phenylpropanoid pathway, and essential oil composition in two Curcuma Species (C. Caesia Roxb. and C. Longa L.). Ecotoxicol. Environ. Saf. 2021, 208, 111739. [Google Scholar] [CrossRef]
- Xue, Y.; Cao, B.; Liang, H.; Yang, J.; Gao, P.; Mao, M.; Li, G.; Bai, C. Environmental shifts have important impacts on the functional traits and bioactive products of medicinal crop Cornus Officinalis. Ind. Crops Prod. 2021, 162, 113304. [Google Scholar] [CrossRef]
- Žarković, B.; Radovanović, V. Environmental impact of climate change on crop production. In Handbook of Climate Change Across the Food Supply Chain; Leal Filho, W., Djekic, I., Smetana, S., Kovaleva, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 321–333. ISBN 9783030879334. [Google Scholar]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A comprehensive review on grain legumes as climate-smartcrops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- Pastore, M.A.; Lee, T.D.; Hobbie, S.E.; Reich, P.B. Strong photosynthetic acclimation and enhanced water-use efficiency in grassland functional groups persist over 21 years of CO2 enrichment, independent of nitrogen supply. Glob. Change Biol. 2019, 25, 3031–3044. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in Tomatoes. Plant Soil 2019, 434, 397–411. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F. Effects of elevated CO2 and heat on wheat grain quality. Plants 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Joshi, R.; Kumari, M.; Thakur, R.; Kumar, D.; Kumar, S. Elevated CO2 and temperature influence key proteins and metabolites associated with photosynthesis, antioxidant and carbon metabolism in Picrorhizakurroa. J. Proteom. 2020, 219, 103755. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chandola, V.; Sultan, Z.; Singh, C.P.; Purohit, V.K.; Nautiyal, B.P.; Nautiyal, M.C. Climate change adversely affects the medicinal value of Aconitum Species in alpine region of Indian Himalaya. Ind. Crops Prod. 2022, 186, 115277. [Google Scholar] [CrossRef]
- Bencke-Malato, M.; De Souza, A.P.; Ribeiro-Alves, M.; Schmitz, J.F.; Buckeridge, M.S.; Alves-Ferreira, M. Short-term responses of soybean roots to individual and combinatorial effects of elevated [CO2] and water deficit. Plant Sci. 2019, 280, 283–296. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Change Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef]
- Stutte, G.W.; Eraso, I.; Rimando, A.M. Carbon dioxide enrichment enhances growth and flavonoid content of two Scutellaria species. J. Am. Soc. Hortic. Sci. 2008, 133, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Biel, C.; Savé, R.; Cristobal, R.; Cases, M.A. effects of atmospheric carbon dioxide concentration on Thymus vulgaris, Thymus zygis and Thymus hyemalis. Acta Hortic. 2005, 676, 61–65. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and Strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Balkhyour, M.A.; Hassan, A.H.A.; Halawani, R.F.; Summan, A.S.; AbdElgawad, H. Effect of elevated co2 on seed yield, essential oil metabolism, nutritive value, and biological activity of Pimpinella anisum L. accessions at different seed maturity stages. Biology 2021, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Misra, G.; Sreenivasulu, N.; Henry, A. What happens at night? Physiological mechanisms related to maintaining grain yield under high night temperature in rice. Plant Cell Environ. 2021, 44, 2245–2261. [Google Scholar] [CrossRef]
- Li, G.; Chen, T.; Feng, B.; Peng, S.; Tao, L.; Fu, G. Respiration, rather than photosynthesis, determines rice yield loss under moderate high-temperature conditions. Front. Plant Sci. 2021, 12, 678653. [Google Scholar] [CrossRef]
- Jochum, G.M.; Mudge, K.W.; Thomas, R.B. Elevated Temperatures Increase Leaf Senescence and Root Secondary Metabolite Concentrations in the Understory Herb Panax Quinquefolius (Araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, R.; Pandey, S.; Chanda, S.; Bhattacharya, A.; Ahuja, P.S. Temperature-dependent growth and emergence of functional leaves: An adaptive mechanism in the seedlings of the western himalayan plant Podophyllum hexandrum. J. Plant Res. 2008, 121, 299–309. [Google Scholar] [CrossRef]
- Hou, M.; Tian, F.; Zhang, T.; Huang, M. Evaluation of canopy temperature depression, transpiration, and canopy greenness in relation to yield of soybean at reproductive stage based on remote sensing imagery. Agric. Water Manag. 2019, 222, 182–192. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Residual transpiration rate, epicuticular wax load and leaf colour of pea plants in drought conditions. Influence on harvest index and canopy temperature. Eur. J. Agron. 2001, 15, 57–70. [Google Scholar] [CrossRef]
- Coast, O.; Posch, B.C.; Bramley, H.; Gaju, O.; Richards, R.A.; Lu, M.; Ruan, Y.; Trethowan, R.; Atkin, O.K. Acclimation of leaf photosynthesis and respiration to warming in field-grown wheat. Plant Cell Environ. 2021, 44, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Bonsu, I.O.; McClain, A.; Walker, B.; Sharkey, T.; Kramer, D.M. The roles of photorespiration and alternative electron acceptors in the responses of photosynthesis to elevated temperatures in cowpea. Plant Cell Environ. 2021, 44, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Elferjani, R.; Soolanayakanahally, R. Canola responses to drought, heat, and combined stress: Shared and specific effects on carbon assimilation, seed yield, and oil composition. Front. Plant Sci. 2018, 9, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahi, P.; Mandal, R.A.; Mathema, A.B. Assessing the impact of climate change on medicinal herbs in Humla district. Int. J. Geogr. Geol. Environ. 2020, 2, 11–17. [Google Scholar]
- Pandey, V.; Tiwari, D.C.; Dhyani, V.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Physiological and metabolic changes in two himalayan medicinal herbs under drought, heat and combined stresses. Physiol. Mol. Biol. Plants 2021, 27, 1523–1538. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.; Turner, N.C. The impact of temperature variability on wheat yields: Impact of temperature variability on wheat yields. Glob. Change Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ran, W.; Jian-Hang, N.; Zohaib, A.; Jin-Huan, L.; Mei-Ru, L.; Ji-Xuan, S.; Jun, L.; San-Gen, W.; Xue-Feng, Z. Exogenous application of ALA regulates growth and physiological characters of Leymus chinensis (Trin.) Tzvel. under low temperature stress. J. Anim. Plant Sci. 2016, 26, 1354–1360. [Google Scholar]
- Liang, J.; Guo, Z.; Ye, Y. Effects of different soil moisture conditions on photosynthetic characteristics and effective content of saponin of Paris polyphylla. Plant Physiol. 2014, 50, 56–60. [Google Scholar]
- Dian-xia, B. Effect of dehydration method on chlorophyll fluorescence characteristics in Vitex trifolia. J. Anhui Agric. Sci. 2010, 6, 168. [Google Scholar]
- Mamnabi, S.; Nasrollahzadeh, S.; Ghassemi-Golezani, K.; Raei, Y. Improving Yield-Related Physiological Characteristics of Spring Rapeseed by Integrated Fertilizer Management under Water Deficit Conditions. Saudi J. Biol. Sci. 2020, 27, 797–804. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Wang, L. Drought tolerance in three maize cultivars is related to differential osmolyte accumulation, antioxidant defense system, and oxidative damage. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [PubMed] [Green Version]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic Acid Improves Drought-Stress Tolerance by Regulating the Redox Status and Proline Metabolism in Brassica rapa. Hortic. Environ. Biotechnol. 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Luo, A.; Chen, Y.; Fan, Y. Drought stress and re-watering increase secondary metabolites and enzyme activity in Dendrobium moniliforme. Ind. Crops Prod. 2016, 94, 385–393. [Google Scholar] [CrossRef]
- Sanjeeta, K.; Parkash, J.; Kalita, P.J.; Devi, M.; Pathania, J.; Joshi, R.; Dutt, S. Comparative proteome analysis of Picrorhiza kurrooa Royle Ex Benth. in response to drought. J. Proteome Sci. Comput. Biol. 2014, 3, 2. [Google Scholar] [CrossRef]
- Asha, A.D.; Nivetha, N.; Krishna, G.K.; Thakur, J.K.; Rathi, M.S.; Manjunatha, B.S.; Chinnusamy, V.; Paul, S. Amelioration of short-term drought stress during different growth stages in Brassica Juncea by Rhizobacteria mediated maintenance of ROS homeostasis. Physiol. Plant. 2021, 172, 1880–1893. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Hossain, A.; Barutçular, C.; Islam, M.S.; Ratnasekera, D.; Kumar, N.; Meena, R.S.; Gharib, H.S.; Saneoka, H.; da Silva, J.A.T. Drought and salinity stress management for higher and sustainable canola (Brassica napus L.) production: A critical review. Aust. J. Crop Sci. 2019, 13, 88–96. [Google Scholar] [CrossRef]
- Mirfattahi, Z.; Karimi, S.; Roozban, M.R. Salinity Induced changes in water relations, oxidative damage and morpho-physiological adaptations of pistachio genotypes in soilless culture. Acta Agric. Slov. 2017, 109, 291. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Van der Kooi, C.J.; Reich, M.; Löw, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated CO2and drought: A meta-analysis on crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plant. 2020, 170, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Feng, B.; Zhang, C.; Yang, Y.; Yang, X.; Chen, T.; Zhao, X.; Zhang, X.; Jin, Q.; Tao, L. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza Sativa L.) due to their different organ temperatures. Front. Plant Sci. 2016, 7, 1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.X.; Fu, G.F.; Yang, X.Q.; Yang, Y.J.; Zhao, X.; Chen, T.T.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J. Agron. Crop Sci. 2016, 202, 394–408. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Tang, T.; Messina, C.D. Response of maize photosynthesis to high temperature: Implications for modeling the impact of global warming. Plant Physiol. Biochem. 2019, 141, 202–205. [Google Scholar] [CrossRef]
- Zheng, S.; Tian, M.; Liu, J.; Zhao, T.; Zhang, Z. Effects of low-light stress on photosynthetic characteristics of Paris polyphylla var. Chinensis in artificial domestication cultivation. J. Chin. Med. Mater. 2014, 37, 1518–1522. [Google Scholar]
- Chaturvedi, A.K.; Vashistha, R.K.; Rawat, N.; Prasad, P.; Nautiyal, M.C. Effect of CO2 Enrichment on photosynthetic behavior of Podophyllum Hexandrum Royle, an endangered medicinal herb. J. Am. Sci. 2009, 5, 113–118. [Google Scholar]
- Singh, H.; Sharma, R.; Verma, A.; Kumar, M.; Kumar, S. Can atmospheric CO2 enrichment alter growth dynamics, structure and functioning of medicinal and aromatic plant (Tulsi) An approach to understand adaptation and mitigation potential of medicinal and aromatic plants in wake of climate change scenario? In Proceedings of the Annual Session of the National Academy of Sciences India, Dehradun, India; 2016; p. 94. [Google Scholar]
- Chang, J.; Mantri, N.; Sun, B.; Jiang, L.; Chen, P.; Jiang, B.; Jiang, Z.; Zhang, J.; Shen, J.; Lu, H.; et al. Effects of elevated CO2 and temperature on Gynostemmapentaphyllum physiology and bioactive compounds. J. Plant Physiol. 2016, 196–197, 41–52. [Google Scholar] [CrossRef]
- Hao, X.; Li, P.; Feng, Y.; Han, X.; Gao, J.; Lin, E.; Han, Y. Effects of fully open-air [CO2] elevation on leaf photosynthesis and ultrastructure of Isatis Indigotica Fort. PLoS ONE 2013, 8, e74600. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F.; McMillan, A.M.S. Warming and elevated CO2 have opposing influences on transpiration. Which is more important? Curr. For. Rep. 2018, 4, 51–71. [Google Scholar] [CrossRef] [Green Version]
- Belko, N.; Zaman-Allah, M.; Cisse, N.; Diop, N.N.; Zombre, G.; Ehlers, J.D.; Vadez, V. Lower soil moisture threshold for transpiration decline under water deficit correlates with lower canopy conductance and higher transpiration efficiency in drought-tolerant cowpea. Funct. Plant Biol. 2012, 39, 306. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Dugo, V.; Lopez-Lopez, M.; Espadafor, M.; Orgaz, F.; Testi, L.; Zarco-Tejada, P.; Lorite, I.J.; Fereres, E. Transpiration from canopy temperature: Implications for the assessment of crop yield in almond orchards. Eur. J. Agron. 2019, 105, 78–85. [Google Scholar] [CrossRef]
- Sigurdsson, B.D. Elevated [CO2] and nutrient status modified leaf phenology and growth rhythm of young Populus trichocarpa trees in a 3-year field study. Trees 2001, 15, 403–413. [Google Scholar] [CrossRef]
- Lee, T.D.; Reich, P.B.; Bolstad, P.V. Acclimation of leaf respiration to temperature is rapid and related to specific leaf area, soluble sugars and leaf nitrogen across three temperate deciduous tree species. Funct. Ecol. 2005, 19, 640–647. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol. 2007, 174, 367–380. [Google Scholar] [CrossRef]
- Chávez-Arias, C.C.; Ligarreto-Moreno, G.A.; Ramírez-Godoy, A.; Restrepo-Díaz, H. Maize responses challenged by drought, elevated daytime temperature and arthropod herbivory stresses: A Physiological, Biochemical and Molecular View. Front. Plant Sci. 2021, 12, 702841. [Google Scholar] [CrossRef]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for Quinoa (Chenopodium Quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Xue, Y.F.; Liu, Z.P. Antioxidant Enzymes and physiological characteristics in two Jerusalem artichoke cultivars under salt stress. Russ. J. Plant Physiol. 2008, 55, 776–781. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef] [PubMed]
- Generozova, I.P.; Butsanets, P.A.; Shugaev, A.G. Mitochondrial respiration after combined action of dehydration and low temperature in pea seedlings. Biol. Plant 2019, 63, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plant Physiology and Development, 6th ed.; Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A.S. (Eds.) Sinauer Associates, Inc.: Sunderland, MA, USA, 2015; ISBN 9781605352558. [Google Scholar]
- Broberg, M.C.; Högy, P.; Feng, Z.; Pleijel, H. Effects of elevated CO2 on wheat yield: Non-linear response and relation to site productivity. Agronomy 2019, 9, 243. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- You, L.; Rosegrant, M.W.; Wood, S.; Sun, D. Impact of growing season temperature on wheat productivity in China. Agric. For. Meteorol. 2009, 149, 1009–1014. [Google Scholar] [CrossRef]
- Yao, F.; Huang, J.; Cui, K.; Nie, L.; Xiang, J.; Liu, X.; Wu, W.; Chen, M.; Peng, S. Agronomic performance of high-yielding rice variety grown under alternate wetting and drying irrigation. Field Crops Res. 2012, 126, 16–22. [Google Scholar] [CrossRef]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I.; Netra Chhetri, N.C.; Garrett, K. Food Security and Food Production Systems; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Yang, X.; Wang, B.; Chen, L.; Li, P.; Cao, C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019, 9, 3742. [Google Scholar] [CrossRef] [Green Version]
- El-Sabagh, A.; Barutcular, C.; Hossain, A.; Islam, M.S. Response of maize hybrids to drought tolerance in relation to grain weight. Fresenius Environ. Bull. 2018, 27, 2476–2482. [Google Scholar]
- Farzi-Aminabad, R.; Ghassemi-Golezani, K.; Nasrullahzadeh, S. Grain and oil yields of Safflower ( Carthamus Tinctorius L.) affected by water deficit and growth regulators. Agriculture 2021, 67, 87–94. [Google Scholar] [CrossRef]
- Vurro, E.; Bruni, R.; Bianchi, A.; Toppi, L. Elevated atmospheric CO2 decreases oxidative stress and increases essential oil yield in leaves of Thymus vulgaris grown in a mini-FACE system. Environ. Exp. Bot. 2009, 65, 99–106. [Google Scholar] [CrossRef]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2018, 163, 516–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, A.M.; Selim, S.; Jaouni, S.A.; AbdElgawad, H. CO2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). Food Chem. 2018, 269, 519–526. [Google Scholar] [CrossRef]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in roundup ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef]
- Albert, A.; Sareedenchai, V.; Heller, W.; Seidlitz, H.K.; Zidorn, C. Temperature is the key to altitudinal variation of phenolics in Arnica montana L. Cv. ARBO. Oecologia 2009, 160, 1–8. [Google Scholar] [CrossRef]
- Nchabeleng, L. Effects of chemical composition of wild bush tea (Athrixia phylicoides DC.) growing at locations differing in altitude, climate and edaphic factors. J. Med. Plants Res. 2012, 6, 1662–1666. [Google Scholar] [CrossRef] [Green Version]
- Kaundal, M.; Bhatt, V.; Kumar, R. Elevated CO2 and temperature effect on essential oil content and composition of Valeriana Jatamansi Jones. with organic manure application in a western Himalayan region. J. Essent. Oil Bear. Plants 2018, 21, 1041–1050. [Google Scholar] [CrossRef]
- Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V.; Jagadish, K.S.V. Agronomic and physiological responses to high temperature, drought, and elevated CO2interactions in cereals. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 127, pp. 111–156. ISBN 9780128001318. [Google Scholar]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07709. [Google Scholar] [CrossRef] [PubMed]
- Patni, B.; Bhattacharyya, M.; Kumari, A.; Purohit, V.K. Alarming influence of climate change and compromising quality of medicinal plants. Plant Physiol. Rep. 2022, 27, 1–10. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Jamloki, A.; Patni, B. Exploring the effects of elevated carbon dioxide mediated global warming phenomenon in photosynthesis: Challenges and future directions. IJGW 2022, 26, 269–293. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Patni, B. Prediction of future world on the basis of high heat conditions on plant metabolism. JSPB 2021, 17, 72–76. [Google Scholar]


| Trait | Species | Change (+/−) | Effect of Change on the Plant (+/−) | Reference |
|---|---|---|---|---|
| Elevated CO2 | ||||
| Photosynthesis | Legumes | + | + | [13] |
| Photosynthetic acclimation | Tomato | + | − | [14] |
| Yield | Wheat, rice | + | + | [15] |
| Levels of PSI and PSII | P. kurroa | + | + | [16] |
| Stomatal conductance | + | + | ||
| Lipid peroxidation | A. balfourii, A. hetrophyllum | + | − | [17] |
| Antioxidant activity | Soybean | + | − | [18] |
| Productivity | Maize; Sorghum | − | − | [19] |
| Growth rate Total biomass | S. barbata, S. lateriflora | + | + | [20] |
| Essential oil content | T. vulgaris, T. hyemalis | + | [21] | |
| Iron assimilatory genes | Soybean | − | − | [18] |
| Nutrient content in edible produce | Wheat, rice, legumes, vegetables | − | [22] | |
| Medicinally important metabolites, vitamins | Herbal plant species | + | [23] | |
| Elevated temperature | ||||
| Photosynthesis | Rice | − | − | [24] |
| Respiration rate | Rice | + | − | [25] |
| Non-structural carbohydrate in stem | Rice | − | − | [26] |
| Activity of NADH dehydrogenase, cytochrome c oxidase, ATPase | Rice | + | + | [27] |
| Photosynthesis | P. quinquefolius | − | − | [28] |
| Transpiration rate | P. hexandrum | + | − | [29] |
| Water use efficiency | − | + | ||
| Canopy temperature depression | Soybean | − | − | [30] |
| Epicuticular wax | Pea | + | + | [31] |
| Dark respiration | Wheat | − | + | [32] |
| Respiration | Cowpea | + | + | [33] |
| Photorespiration | + | + | ||
| Electron transport rate | B. napus | − | − | [34] |
| Carboxylation efficiency | − | − | ||
| Oil quality | − | − | ||
| Seed yield | − | − | ||
| Growth and multiplication | P. polyphylla, S. chirayita | + | [35] | |
| Linalool concentration | H. spicatum | + | [36] | |
| Low temperature | ||||
| Mitochondrial respiration | Pea | + | − | [37] |
| Malondialdehyde content | Rye grass (L. chinensis) | + | − | [38] |
| Osmolytes | + | + | ||
| Antioxidants | + | + | ||
| Drought | ||||
| Chlorophyll content | P. polyphylla | − | − | [39] |
| Light compensation point | + | − | ||
| Chlorophyll fluorescence | V. trifolia | + | − | [40] |
| PSII activity | − | − | ||
| Membrane stability index | Rapeseed | − | − | [41] |
| Malondialdehyde content | + | − | ||
| ROS accumulation | Maize hybrids | + | − | [42] |
| Oxidized/reduced glutathione ratio | Chinese cabbage | − | − | [43] |
| NADP/NADPH ratio | − | − | ||
| CAT and POD activities | D. moniliforme | + | + | [44] |
| Leaf and root protein content | Picrorhiza sp. | − | − | [45] |
| ROS homeostasis | Mustard | − | − | [46] |
| Salinity | ||||
| Biomass and oil yield | Canola | − | − | [47] |
| Membrane stability index | Pistachio | − | − | [48] |
| Malondialdehyde content | + | − | ||
| Elevated CO2 + temperature | ||||
| Yield | Wheat, rice | − | − | [15] |
| Drought + temperature | ||||
| Photosynthetic rate | H. spicatum, V. jatamansi | − | − | [36] |
| Valerenic acid content | V. jatamansi | − | ||
| Molecule Name | Formula | Half-Life |
|---|---|---|
| Non-radicals | ||
| Hydrogen peroxide | H2O2 | Stable |
| Singlet oxygen | 1O2 | 10−6 s |
| Ozone | O3 | Stable |
| Organic peroxide | ROOH | Stable |
| Hypochlorous acid | HOCl | Stable (min) |
| Hypobromous acid | HOBr | Stable (min) |
| Free radicals | ||
| Superoxide | O2•− | 10−6 s |
| Hydroxyl radical | OH• | 10−10 s |
| Alkoxyl radical | RO• | 10−6 s |
| Peroxyl Radical | ROO• | 17 s |
| Nitric oxide | NO• | 2 ms |
| Nitrogen dioxide | NO2• |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, A.; Lakshmi, G.A.; Krishna, G.K.; Patni, B.; Prakash, S.; Bhattacharyya, M.; Singh, S.K.; Verma, K.K. Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture. Agronomy 2022, 12, 3008. https://doi.org/10.3390/agronomy12123008
Kumari A, Lakshmi GA, Krishna GK, Patni B, Prakash S, Bhattacharyya M, Singh SK, Verma KK. Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture. Agronomy. 2022; 12(12):3008. https://doi.org/10.3390/agronomy12123008
Chicago/Turabian StyleKumari, Aradhna, Geetha Ajay Lakshmi, Gopinathan Kumar Krishna, Babita Patni, Soban Prakash, Malini Bhattacharyya, Santosh Kumar Singh, and Krishan Kumar Verma. 2022. "Climate Change and Its Impact on Crops: A Comprehensive Investigation for Sustainable Agriculture" Agronomy 12, no. 12: 3008. https://doi.org/10.3390/agronomy12123008