Effect of Long-Term Soil Management Practices on Tree Growth, Yield and Soil Biodiversity in a High-Density Olive Agro-Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Fruit Yield, Trunk Growth and Root Distribution
2.3. Soil Physical and Chemical Properties
2.4. Edaphic Arthropod Community and Functional Biodiversity
2.5. Statistical Analysis
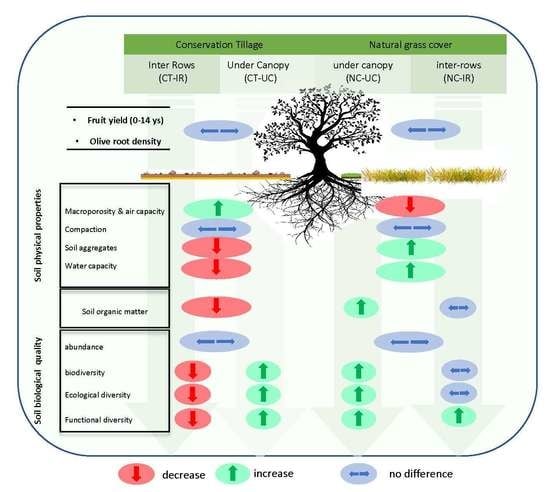
3. Results
3.1. Fruit Yield, Trunk Growth and Root Distribution
3.2. Soil Physical and Chemical Properties
3.3. Characterization of Edaphic Arthropod Community and Soil Biodiversity
3.4. Functional Biodiversity of Edaphic Arthropods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| CT-IR | CT-UC | NC-IR | NC-UC | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Animal Group | I | II | III | IV | V | VI | Tot | I | II | III | IV | V | VI | Tot | I | II | III | IV | V | VI | Tot | I | II | III | IV | V | VI | Tot |
| Aracnida | Acari | 115 | 47 | 188 | 127 | 219 | 66 | 762 | 71 | 319 | 595 | 956 | 508 | 490 | 2939 | 87 | 361 | 423 | 420 | 132 | 335 | 1758 | 160 | 304 | 496 | 366 | 306 | 198 | 1830 |
| Araneida | 1 | 1 | 2 | 1 | 5 | 5 | 3 | 8 | 1 | 1 | 2 | 1 | 1 | 6 | |||||||||||||||
| Pseudoscorpionida | 1 | 1 | 2 | 2 | 2 | ||||||||||||||||||||||||
| Entognatha | Collembola | 18 | 7 | 157 | 70 | 116 | 54 | 422 | 12 | 210 | 92 | 239 | 218 | 154 | 925 | 93 | 325 | 403 | 278 | 271 | 1370 | 59 | 358 | 920 | 721 | 81 | 5 | 2144 | |
| Protura | 1 | 1 | 1 | 1 | 4 | 1 | 4 | 9 | 6 | 20 | 8 | 4 | 3 | 3 | 4 | 1 | 23 | ||||||||||||
| Diplura | 2 | 9 | 18 | 1 | 6 | 36 | 1 | 33 | 2 | 7 | 3 | 46 | 27 | 27 | 46 | 58 | 1 | 1 | 160 | ||||||||||
| Crustacea | Isopoda | 2 | 1 | 3 | 7 | 2 | 2 | 2 | 13 | ||||||||||||||||||||
| Myriapoda | Symphyla | 1 | 1 | 4 | 3 | 1 | 2 | 10 | 3 | 41 | 8 | 1 | 4 | 57 | 2 | 6 | 10 | 7 | 10 | 2 | 37 | ||||||||
| Geophylomorpha | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 1 | 2 | 2 | 12 | |||||||||||||||||
| Lithobiomorpha | 1 | 1 | 2 | 1 | 1 | ||||||||||||||||||||||||
| Pauropoda | 1 | 1 | 4 | 2 | 4 | 12 | 6 | 2 | 1 | 1 | 2 | 12 | 8 | 49 | 9 | 24 | 10 | 100 | 1 | 19 | 5 | 24 | 63 | 112 | |||||
| Polyxenida | 2 | 2 | 5 | 3 | 8 | ||||||||||||||||||||||||
| Julida | 1 | 3 | 12 | 10 | 6 | 32 | 2 | 2 | 4 | 5 | 6 | 4 | 1 | 12 | 28 | ||||||||||||||
| Insecta | Thysanoptera | 1 | 1 | 2 | 4 | 1 | 1 | 1 | 1 | 2 | 2 | ||||||||||||||||||
| Diptera larvae | 7 | 11 | 11 | 10 | 27 | 66 | 11 | 6 | 4 | 36 | 11 | 68 | 14 | 37 | 11 | 18 | 80 | 4 | 11 | 14 | 34 | 3 | 66 | ||||||
| Embioptera | 1 | 1 | |||||||||||||||||||||||||||
| Heteroptera | 1 | 1 | 2 | 27 | 2 | 29 | 1 | 1 | 5 | 7 | |||||||||||||||||||
| Hymenoptera | 2 | 2 | 1 | 8 | 9 | 1 | 1 | ||||||||||||||||||||||
| Formicidae | 5 | 1 | 2 | 8 | 1 | 22 | 16 | 39 | 23 | 7 | 121 | 26 | 7 | 184 | 19 | 8 | 3 | 1 | 13 | 1 | 45 | ||||||||
| Lepidoptera larvae | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| Coleoptera | 4 | 1 | 1 | 1 | 7 | 1 | 2 | 3 | 6 | ||||||||||||||||||||
| Coleoptera larvae | 1 | 1 | 1 | 3 | 6 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 5 | 2 | 1 | 1 | 2 | 6 | ||||||||||
| Psocoptera | 1 | 2 | 1 | 4 | |||||||||||||||||||||||||

References
- FAO. The State of Food and Agriculture Trends and Challenges. 2017. Available online: http://www.fao.org/3/a-i6583e.pdf (accessed on 3 March 2021).
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2019, 178, 102736. [Google Scholar] [CrossRef]
- Ramos, M.E.; Benítez, E.; García, P.A.; Robles, A.B. Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: Effects on soil quality. Agric. Ecosyst. Environ. Appl. Soil Ecol. 2010, 44, 6–14. [Google Scholar] [CrossRef]
- Garcia, C.; Roldan, A.; Hernandez, T. Ability of different plant species to promote microbiological processes in semiarid soil. Geoderma 2005, 124, 193–202. [Google Scholar] [CrossRef]
- Terribile, F.; Basile, A.; Bonfante, A.; Carbone, A.; Colombo, C.; Langella, G.; Iamarino, M.; Manna, P.; Minieri, L.; Vingiani, S. Future Soil Issues. In The Soils of Italy; Costantini, E., Dazzi, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 303–348. [Google Scholar] [CrossRef]
- Bordoni, M.; Vercesi, A.; Maerker, M.; Ganimede, C.; Reguzzi, M.C.; Capelli, E.; Wei, X.; Mazzoni, E.; Simoni, S.; Gagnarli, E.; et al. Effects of vineyard soil management on the characteristics of soils and roots in the lower Oltrepò Apennines (Lombardy, Italy). Sci. Total Environ. 2019, 693, 133390. [Google Scholar] [CrossRef] [PubMed]
- Corti, G.; Cocco, S.; Brecciaroli, G.; Agnelli, A.; Seddaiu, G. Italian Soil Management from Antiquity to Nowadays. In The Soils of Italy; Costantini, E., Dazzi, C., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 247–294. [Google Scholar] [CrossRef]
- Gómez, J.A.; Romero, P.; Giraldez, J.V.; Fereres, E. Experimental assessment of runoff and soil erosion in an olive grove on a Vertic soil in southern Spain as affected by soil management. Soil. Use Manag. 2004, 20, 426–431. [Google Scholar] [CrossRef]
- Gucci, R.; Caruso, G.; Bertolla, C.; Urbani, S.; Taticchi, A.; Esposto, S.; Servili, M.; Sifola, M.I.; Pellegrini, S.; Pagliai, M.; et al. Changes of soil properties and tree performance induced by soil management in a high-density olive orchard. Eur. J. Agron. 2012, 41, 18–27. [Google Scholar] [CrossRef]
- Gómez, J.A.; Giraldez, J.V.; Pastor, M.; Fereres, E. Effects of tillage method on soil physical properties, infiltration and yield in an olive orchard. Soil Till. Res. 1999, 52, 167–175. [Google Scholar] [CrossRef]
- Hernández, A.J.; Lacasta, C.; Pastor, J. Effects of different management practices on soil conservation and soil water in a rainfed olive orchard. Agric. Water Manag. 2005, 77, 232–248. [Google Scholar] [CrossRef]
- Atucha, A.; Merwin, I.A.; Brown, M.G. Long-term effects of four groundcover management systems in an apple orchard. Hort. Sci. 2011, 46, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Dichio, B.; Romano, M.; Nuzzo, V.; Xiloyannis, C. Soil water availability and relationship between canopy and roots in young olive trees (cv coratina). Acta Hortic. 2002, 586, 251–254. [Google Scholar] [CrossRef]
- Tognetti, R.; Morales-Sillero, A.; D’Andria, R.; Fernández, J.E.; Lavini, A.; Sebastiani, L.; Troncoso, A. Deficit irrigation and fertigation practices in olive growing: Convergences and divergences in two case studies. Plant Biosyst. 2008, 142, 138–148. [Google Scholar] [CrossRef]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef]
- Vignozzi, N.; Agnelli, A.E.; Brandi, G.; Gagnarli, E.; Lagomarsino, A.; Pellegrini, S.; Simoncini, S.; Simoni, S.; Valboa, G.; Gucci, R. Soil ecosystem functions in a high-density olive orchard managed by different soil conservation practices. Appl. Soil Ecol. 2019, 134, 64–76. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Mudgal, S.; Turbé, A.; de Toni, A.; Lavelle, P.; Benito, P.; Ruiz, N. Soil Biodiversity: Functions, Threats and Tools for Policy Makers; Bio Intelligence Service, IRD, and NIOO Report for European Commission; 2010; p. 249. Available online: http://ec.europa.eu/environment/archives/soil/pdf/biodiversity_report.pdf (accessed on 3 March 2021).
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Neher, D.A.; Barbercheck, M.E. Soil Microarthropods and Soil Health: Intersection of Decomposition and Pest Suppression in Agroecosystems. Insects 2019, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, U.-N. Approaches to Studying Soil Fauna and Its Functional Roles. In Soil Fauna Assemblages: Global to Local Scales (Ecology, Biodiversity and Conservation); Cambridge University Press: Cambridge, UK, 2019; pp. 86–120. [Google Scholar] [CrossRef]
- Bispo, A.; Cluzeau, D.; Creamer, R.; Dombos, M.; Graefe, U.; Krogh, P.; Sousa, J.; Peres, G.; Rutgers, M.; Winding, A.; et al. Indicators for monitoring soil biodiversity. Integr. Environ. Assess. Manag. 2009, 5, 717–719. [Google Scholar] [CrossRef] [Green Version]
- Culliney, T.W. Role of arthropods in maintaining soil fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef] [Green Version]
- Menta, C.; Conti, F.D.; Fondón, C.L.; Staffilani, F.; Remelli, S. Soil arthropod responses in agroecosystem: Implications of different management and cropping systems. Agronomy 2020, 10, 982. [Google Scholar] [CrossRef]
- Lamarre, G.P.A.; Hérault, B.; Fine, P.V.A.; Vedel, V.; Lupoli, R.; Mesones, I.; Baraloto, C. Taxonomic and functional composition of arthropod assemblages across contrasting Amazonian forests. J. Anim. Ecol. 2016, 85, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil Macrofauna: A key Factor for Increasing Soil Fertility and Promoting Sustainable Soil Use in Fruit Orchard Agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Menta, C.; Remelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- Garcia-Torres, L.; Martinez-Vilela, A.; Serrano De Norena, F. Conservation agriculture in Europe: Current status and perspectives. In Conservation Agriculture; García-Torres, L., Benites, J., Martínez-Vilela, A., Holgado-Cabrera, A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 87–91. [Google Scholar] [CrossRef]
- Beaufoy, G. The Environmental Impact of Olive Oil Production in the European Union: Practical Options for Improving the Environmental Impact. Eur. Com. 2000, 1–73. [Google Scholar]
- Menta, C. Soil Fauna Diversity-Function, Soil Degradation, Biological Indices, Soil Restoration. In Biodiversity Conservation and Utilization in a Diverse World; Gbolagade, A.L., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Seastedt, T.R. The role of microarthropods in decomposition and mineralization processes. Annu. Rev. Entomol. 1984, 29, 25–46. [Google Scholar] [CrossRef]
- Brussaard, L. Soil fauna, guilds, functional groups and ecosystem processes. Appl. Soil Ecol. 1998, 9, 123–135. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, J.A.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Elsevier Academic Press: Burlington, MA, USA, 2004; p. 408. [Google Scholar]
- Bedano, J.C.; Domínguez, A.; Arolfo, R. Assessment of soil biological degradation using mesofauna. Soil Tillage Res. 2011, 117, 55–60. [Google Scholar] [CrossRef]
- Caruso, T.; Pigino, G.; Bernini, F.; Bargagli, R.; Migliorini, M. The Berger-Parker index as an effective tool for monitoring the biodiversity of disturbed soils: A case study on Mediterranean oribatid (Acari: Oribatida) assemblages. Biodivers. Conserv. 2007, 16, 3277–3285. [Google Scholar] [CrossRef]
- Behan-Pelletier, V.M. Acari and collembola biodiversity in Canadian agricultural soils. Can. J. Soil Sci. 2003, 83, 279–288. [Google Scholar] [CrossRef]
- Maaß, S.; Caruso, T.; Rillig, M.C. Functional role of microarthropods in soil aggregation. Pedobiologia 2015, 58, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Castaño-Meneses, G.; Palacios-Vargas, J.G.; Cutz-Pool, L.Q. Feeding habits of Collembola and their ecological niche. An. Inst. Biol. Univ. Nac. Autónoma México Ser. Zool. 2004, 75, 135–142. [Google Scholar]
- Moore, J.C.; Berlow, E.L.; Coleman, D.C.; de Ruiter, P.C.; Dong, Q.; Hastings, A.; Collins Johnson, N.; McCann, K.S.; Melville, K.; Morin, P.J.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Brose, U.; Hillebrand, H. Biodiversity and ecosystem functioning in soil. Phil. Trans. R. Soc. B 2016, 371, 267. [Google Scholar] [CrossRef] [Green Version]
- Geisen, S.; Briones, M.J.I.; Gan, H.; Behan-Pelletier, V.M.; Friman, V.-P.; de Groot, G.A.; Hannula, S.E.; Lindo, Z.; Philippot, L.; Tiunov, A.V.; et al. A methodological framework to embrace soil biodiversity. Soil Biol. Bioch. 2019, 136, 107536. [Google Scholar] [CrossRef]
- Caruso, G.; Rapoport, H.F.; Gucci, R. Long-term evaluation of yield components of young olive trees during the onset of fruit production under different irrigation regimes. Irrig. Sci. 2013, 31, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Soil Survey Laboratory Information Manual; Soil Survey Investigations Report No. 45, Version 2.0; Burt, R., Ed.; U.S. Department of Agriculture, Natural Resources Conservation Service: Lincoln, NE, USA, 2011; p. 506. [Google Scholar]
- Cirilli, M.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. The Role of Polyphenoloxidase, Peroxidase, and β-Glucosidase in Phenolics Accumulation in Olea europaea L. Fruits under Different Water Regimes. Front. Plant Sci. 2017, 8, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gucci, R.; Caruso, G.; Gennai, C.; Esposto, S.; Urbani, S.; Servili, M. Fruit growth, yield and oil quality changes induced by deficit irrigation at different stages of olive fruit development. Agric. Water Manag. 2018, 212, 88–98. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Agronomy Monographs 9; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Agronomy Monographs 9; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Pagliai, M. Soil porosity aspects. Int. Agrophys. 1988, 4, 215–232. [Google Scholar]
- Bouma, J.; Jongerius, A.; Boersma, O.H.; Jager, A.; Schoonderbeek, D. Function of different types of macropores during saturated flow through four swelling soil horizons. Sci. Soc. Am. J. 1977, 41, 945–950. [Google Scholar] [CrossRef]
- Greenland, D.J. Soil damage by intensive arable cultivation: Temporary or permanent? Philos. Tran. R. Soc. Lon. 1977, 281, 193–208. [Google Scholar] [CrossRef]
- Clement, C.R. A simple and reliable tension table. J. Soil Sci. 1966, 17, 133–135. [Google Scholar] [CrossRef]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity: Laboratory methods. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Agronomy Monographs 9; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 687–734. [Google Scholar]
- Gardner, G.H.F.; Gardner, L.W.; Gregory, A.R. Formation velocity and density—the diagnostic basics for stratigraphic traps. Geophysics 1974, 39, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, J.C.; Bremner, M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Sequi, P.; De Nobili, M. Frazionamento del carbonio organico. In Metodi di Analisi Chimica del Suolo; Violante, P., Ed.; Franco Angeli: Rome, Italy, 2000; pp. 1–13. [Google Scholar]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Bachelier, G. La Vie Animale Dans le Sol; O.R.S.T.O.M.: Paris, France, 1978; p. 285. [Google Scholar]
- Aoki, J. Difference in sensitivities of oribatid families to environmental change by human impacts. Rev. d’Ecologie Biol. Sol. 1979, 16, 415–422. [Google Scholar]
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (1748–2002). Graellsia 2004, 60, 3–305. [Google Scholar] [CrossRef] [Green Version]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009; p. 816. [Google Scholar]
- Dindal, D.L. Soil Biology Guide; Wiley Interscience: New York, NY, USA, 1990; p. 1376. [Google Scholar]
- Maraun, M.; Heethoff, M.; Schneider, K.; Scheu, S.; Weigmann, G.; Cianciolo, J.; Thomas, R.H.; Norton, R.A. Molecular phylogeny of oribatid mites (Oribatida, Acari): Evidence for multiple radiations of parthenogenetic lineages. Exp. Appl. Acarol. 2004, 33, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K. Transformation using (x + 0.5) to stabilize the variance of populations. Res. Popul. Ecol. 1999, 41, 229–234. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; p. 852. [Google Scholar]
- Gonsalves, M.F.; Pereira, J.A. Abundance and diversity of soil arthropods in the olive grove ecosystem. J. Insect Sci. 2012, 12, 20. [Google Scholar] [CrossRef]
- Gkisakis, V.D.; Kollaros, D.; Bàrberi, P.; Livieratos, I.C.; Kabourakis, E.M. Soil Arthropod Diversity in Organic, Integrated, and Conventional Olive Orchards and Different Agroecological Zones in Crete, Greece. Agroecol. Sustain. Food Syst. 2015, 39, 276–294. [Google Scholar] [CrossRef]
- Buchholz, J.; Querner, P.; Paredes, D.; Bauer, T.; Strauss, P.; Guernion, M.; Scimia, J.; Cluzeau, D.; Burel, F.; Kratschmer, S.; et al. Soil biota in vineyards are more influenced by plants and soil quality than by tillage intensity or the surrounding landscape. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiera, C.; Ulrich, W.; Popescu, D.; Bunea, C.-I.; Manu, M.; Nae, I.; Stan, M.; Markó, B.; Urák, I.; Giurginca, A.; et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in Central Romania. J. Insect Conserv. 2020, 24, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubie, T.R. Effects of Tillage as a Disturbance on Soil Microarthropods and Entomopathogenic Nematodes in Oklahoma. Master’s Thesis, Oklahoma State University, Stillwater, OK, USA, 2010. [Google Scholar]
- Hoffmann, W.A.; Jackson, R.B. Vegetation–climate feedbacks in the conversion of tropical savanna to grassland. J. Clim. 2000, 13, 1593–1602. [Google Scholar] [CrossRef]
- Da Silva, R.F.; de Aquino, A.M.; Mercante, M.F.; Guimarães, M.d.F. Macrofauna invertebrada do solo sob diferentes sistemas de produção em Latossolo da Região do Cerrado. Pesqui. Agropecuária Bras. 2006, 41, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Palla, M.; Turrini, A.; Cristani, C.; Caruso, G.; Avio, L.; Giovannetti, M.; Agnolucci, M. Native mycorrhizal communities of olive tree roots as affected by protective green cover and soil tillage. Appl. Soil Ecol. 2020, 149, 103520. [Google Scholar] [CrossRef]
- Tugel, A.J.; Lewandowski, A.M.; Happe-vonArb, D. Soil Biology Primer; Soil and Water Conservation Society: Ankeny, IA, USA, 2000. [Google Scholar]
- Lavelle, P.; Spain, A.; Blouin, M.; Brown, G.; Decaëns, T.; Grimaldi, M.; Jiménez, J.J.; McKey, D.; Mathieu, J.; Velasquez, E.; et al. Ecosystem engineers in a self-organized soil: A review of concepts and future research questions. Soil Sci. 2016, 181, 91–109. [Google Scholar] [CrossRef] [Green Version]
- Vanhée, B.; Devigne, C. Differences in collembola species assemblages (Arthropoda) between spoil tips and surrounding environments are dependent on vegetation development. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Eisenbeis, G.; Wichard, W. Atlas of the Biology of Soil Arthropods; Springer: Berlin/Heidelberg, Germany, 1987; p. 473. [Google Scholar] [CrossRef]
- Bolico, C.F.; Oliveira, E.A.; Gantes, M.L.; Dumont, L.F.C.; Carrasco, D.S.; D’Incao, F. Mirmecofauna (Hymenoptera, Formicidae) de Duas Marismas do Estuário da Lagoa dos Patos, RS: Diversidade, Flutuação de Abundância e Similaridade como Indicadores de Conservação. EntomoBrasilis 2012, 5, 11–20. [Google Scholar] [CrossRef] [Green Version]
- De Groot, G.A.; Jagers op Akkerhuis, G.A.J.M.; Dimmers, W.J.; Charrier, X.; Faber, J.H. Biomass and diversity of soil mite functional groups respond to extensification of land management, potentially affecting soil ecosystem services. Front. Environ. Sci. 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Helgason, T.; Daniell, T.J.; Husband, R.; Fitter, A.H.; Young, J.P. Ploughing up the wood-wide-web? Nature 1998, 394, 431. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, M.; Pigino, G.; Caruso, T.; Fanciulli, P.P.; Leonzio, C.; Bernini, F. Soil communities (Acari Oribatida; Hexapoda Collembola) in a clay pigeon shooting range. Pedobiologia 2005, 49, 1–13. [Google Scholar] [CrossRef]
- Sánchez-Moreno, S.; Castro, J.; Alonso-Prados, E.; Alonso-Prados, J.-L.; García-Baudín, J.-M.; Talavera, M.; Durán-Zuazo, V.-H. Tillage and herbicide decrease soil biodiversity in olive orchards. Agron. Sustain. Dev. 2015, 35, 691–700. [Google Scholar] [CrossRef]









| Years after Planting | Soil Management | Cumulative Fruit Yield (kg Tree−1) | Cumulative Pruning Weight (kg Tree−1) | ΔTCSA (dm−2) | Fruit Yield/Pruning Weight |
|---|---|---|---|---|---|
| 0–3 (training) | NC | 2.2 ± 2.97 b | - | 0.15 ± 0.03 b | - |
| CT | 6.8 ± 5.44 a | - | 0.25 ± 0.08 a | - | |
| 4–7 (onset of production) | NC | 33.1 ± 9.20 b | 16.2 ± 5.51 b | 1.02 ± 0.15 b | 2.23 ± 1.05 |
| CT | 57.3 ± 16.27 a | 23.4 ± 10.35 a | 1.22 ± 0.23 a | 2.61 ± 0.49 | |
| 8–14 (full production) | NC | 100.2 ± 6.18 | 57.9 ±4.10 b | 1.59 ± 0.15 | 1.78 ± 0.13 |
| CT | 117.5 ± 7.00 | 77.4 ± 6.49 a | 1.51 ± 0.13 | 1.64 ± 0.21 | |
| 0–14 (whole period) | NC | 139.5 ± 7.17 b | 73.7 ± 3.96 b | 2.93 ± 0.17 | 1.91 ± 0.08 |
| CT | 188.7 ± 7.73 a | 104.2 ± 7.09 a | 3.26 ± 0.18 | 1.90 ± 0.19 |
| Management | Position | BD g cm−3 | MWDdry mm | MWDwet mm | AC m3 m−3 | AWC m3 m−3 | TOC % | HC % | DH % |
|---|---|---|---|---|---|---|---|---|---|
| NC | UC | 1.39 (±0.08) | 11.0 a (±0.27) | 6.7 a (±0.11) | 0.16 b (±0.03) | 0.18 a (±1*10−2) | 2.0 a (±0.12) | 0.51 a (±0.06) | 49.9 (±6.33) |
| IR | 1.44 (±0.10) | 12.1 a (±0.18) | 6.6 a (±0.08) | 0.13 b (±0.05) | 0.17 ab (±2*10−3) | 1.9 a (±0.13) | 0.41 ab (±0.10) | 37.3 (±6.84) | |
| CT | UC | 1.23 (±0.04) | 6.4 b (±0.61) | 5.3 b (±0.37) | 0.27 a (±0.01) | 0.16 bc (±3*10−3) | 1.7 a (±0.08) | 0.36 b (±0.07) | 37.1 (±6.00) |
| IR | 1.36 (±0.02) | 6.9 b (±1.03) | 1.7 c (±0.13) | 0.29 a (±0.01) | 0.14 c (±5*10−4) | 1.4 b (±0.04) | 0.20 b (±0.04) | 32.8 (±5.60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simoni, S.; Caruso, G.; Vignozzi, N.; Gucci, R.; Valboa, G.; Pellegrini, S.; Palai, G.; Goggioli, D.; Gagnarli, E. Effect of Long-Term Soil Management Practices on Tree Growth, Yield and Soil Biodiversity in a High-Density Olive Agro-Ecosystem. Agronomy 2021, 11, 1036. https://doi.org/10.3390/agronomy11061036
Simoni S, Caruso G, Vignozzi N, Gucci R, Valboa G, Pellegrini S, Palai G, Goggioli D, Gagnarli E. Effect of Long-Term Soil Management Practices on Tree Growth, Yield and Soil Biodiversity in a High-Density Olive Agro-Ecosystem. Agronomy. 2021; 11(6):1036. https://doi.org/10.3390/agronomy11061036
Chicago/Turabian StyleSimoni, Sauro, Giovanni Caruso, Nadia Vignozzi, Riccardo Gucci, Giuseppe Valboa, Sergio Pellegrini, Giacomo Palai, Donatella Goggioli, and Elena Gagnarli. 2021. "Effect of Long-Term Soil Management Practices on Tree Growth, Yield and Soil Biodiversity in a High-Density Olive Agro-Ecosystem" Agronomy 11, no. 6: 1036. https://doi.org/10.3390/agronomy11061036