Addition of Phosphatases and Phytases to Mature Compost to Increase Available Phosphorus: A Short Study
Abstract
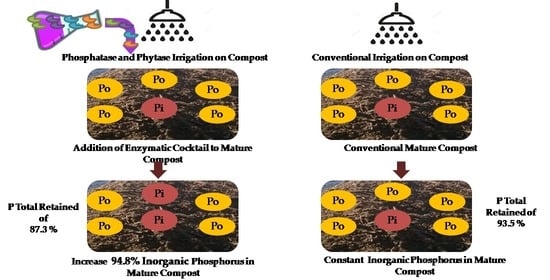
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Enzyme Determination
2.2. Composting Preparation
2.3. Assesment of Phosphate-Solubilizing Activity of Enzymes, Bacteria and Combinations to Increase Pi on Mature Compost
2.4. Assesment of PhoEnz in Higher Percentages to Increase Pi on Mature Compost
2.5. Statistical Analysis
3. Results
3.1. Bacterial Count and Specific Activity
3.2. Phosphate-Solubilizing Activity ofEnzymes, Bacteria and Combination to Mature Compost
3.3. PhoEnz in Higher Percentages to Increase Pi on Mature Compost
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; Neset, T.S.S. Phosphorus vulnerability: A qualitative framework for assessing the vulnerability of national and regional food systems to the multi-dimensional stressors of phosphorus scarcity. Glob. Environ. Chang. 2014, 24, 108–122. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Mayer, B.; Westerhof, P.; Edwards, M. Capturing the lost phosphorus. Chemosphere 2011, 84, 846–853. [Google Scholar] [CrossRef]
- Childers, D.; Corman, J.; Edwards, M.; Elser, J.J. Sustainability challenges of phosphorus and food: Solutions from closing the human phosphorus cycle. BioScience 2011, 61, 117–124. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Z.; Cao, Z.; Zhao, Y.; Zhao, X.; Lu, Q.; Wang, X.; Zhang, X. A regulating method for the distribution of phosphorus fractions based on environmental parameters related to the key phosphate-solubilizing bacteria during composting. Bioresour. Technol. 2016, 211, 610–617. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.; Idiris, A. Cationic and anionic dye adsorption by agricultural solidwastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphorus acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Safari, M.; Motamedi, E.; Kari, A.S.; Modarres, M. Nano-carriers effects on the viability and ef fi ciency of Pseudomonas strains as phosphate solubilizing bacteria. Heliyon 2020, 6, e05076. [Google Scholar] [CrossRef]
- Feng, C.; Zeng, G.; Huang, D.; Hu, S.; Zhao, M.; Lai, C.; Huang, C.; Wei, Z.; Li, N. Effect of ligninolytic enzymes on lignin degradation and carbon utilization during lignocellulosic waste composting. Process. Biochem. 2011, 46, 1515–1520. [Google Scholar] [CrossRef]
- Frossard, E.; Achat, D.L.; Bernasconi, S.M.; Bünemann, E.S.; Fardeau, J.-C.; Jansa, J.; Morel, C.; Rabeharisoa, L.; Randriamanantsoa, L.; Sinaj, S.; et al. The Use of Tracers to Investigate Phosphate Cycling in Soil—Plant Systems. In Phosphorus in Action, Soil Biology; Bunemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e0317. [Google Scholar] [CrossRef] [PubMed]
- Menez-Blackburn, D.; Inostroza, N.G.; Gianfreda, L.; Greiner, R.; Mora, M.L.; Jorquera, M.A. Phytase-producing Bacillus sp. inoculation increases phosphorus availability in cattle manure. J. Soil Sci. Plant Nutr. 2016, 16, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; He, X.; Xi, B.; Gao, R.; Tan, W.; Zhang, H.; Li, D. The evolution of water extractable organic matter and its association with microbial community dynamics during municipal solid waste composting. Waste Manag. 2016, 56, 79–87. [Google Scholar] [CrossRef]
- Turner, B.L.; Engelbrecht, B.M.J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Rocky-salimi, K.; Hashemi, M.; Safari, M. A novel phytase characterized by thermostability and high pH tolerance from rice phyllosphere isolated Bacillus subtilis BS 46. J. Adv. Res. 2016, 7, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thaoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratiasp.isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Co-inoculation with phosphate-solubilizing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur. J. Soil Biol. 2012, 50, 112–117. [Google Scholar] [CrossRef]
- Datta, A.; Gujre, N.; Gupta, D.; Agnihotri, R.; Mitra, S. Application of enzymes as a diagnostic tool for soils as affected by municipal solid wastes. J. Environ. Manag. 2021, 286, 112169. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.Y.; Ma, Y.M.; Tian, X.P. Effects of long-term culture and fertilization on the contents of forms of phosphorus and their availability in albic soil. Acta Agron. Sin. 2005, 31, 48–52. [Google Scholar]
- Mlaik, N.; Khoufi, S.; Hamza, M.; Masmoudi, M.A.; Sayadi, S. Enzymatic pre-hydrolysis of organic fraction of municipal solid waste to enhance anaerobic digestion. Biomass Bioenergy 2019, 127, 105286. [Google Scholar] [CrossRef]
- Tabatai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Paratesh, F.; Alikhani, H.A.; Etesami, H. Vermicomposting enriched with phosphate-solubilizing bacteria provides plant with enough phosphorus in a sequential cropping under calcareous soil conditions. J. Clean. Prod. 2019, 221, 27–37. [Google Scholar] [CrossRef]
- Nash, D.M.; Haygarth, P.M.; Turner, B.L.; Condron, L.M.; McDowell, R.W.; Richardson, A.E.; Watkins, M.; Heaven, M.W. Using organic phosphorus to sustain pasture productivity: A perspective. Geoderma 2014, 221–222, 11–19. [Google Scholar] [CrossRef]
- Annaheim, K.E.; Doolette, A.L.; Smernik, R.J.; Mayer, J.; Oberson, A.; Frossard, E.; Bünemann, E.K. Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma 2015, 257–258, 67–77. [Google Scholar] [CrossRef]
- Torres, I.F.; Bastida, F.; Hernández, T.; García, C. The effects of fresh and stabilized pruning wastes on the biomass, structure and activity of the soil microbial community in a semiarid climate. Appl. Soil Ecol. 2015, 89, 1–9. [Google Scholar] [CrossRef]
- Tyagi, M.; da Fonseca, M.M.R.; de Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2011, 22, 231–241. [Google Scholar] [CrossRef]
- Masy, T.; Demanèche, S.; Tromme, O.; Thonart, P.; Jacques, P.; Hiligsmann, S.; Vogel, T.M. Hydrocarbon biostimulation and bioaugmentation in organic carbon and clay-rich soils. Soil Biol. Biochem. 2016, 99, 66–74. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Mahsefat, M.; Farhangi, M.B.; Rad, M.K.; Proietti, P. Short-term impacts of pomace application and Pseudomonas bacteria on soil available phosphorus. Biocatal. Agric. Biotechnol. 2020, 28, 101742. [Google Scholar] [CrossRef]
- Martínez-Salgado, M.M.; Blu, R.O.; Janssens, M.; Fincheira, P. Grape pomace compost as a source of organic matter: Evolution of quality parameters to evaluate maturity and stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]


| Physicochemical Composition | Values (%) |
|---|---|
| Total nitrogen | 1.955 |
| Total phosphorus | 0.790 |
| Carbon/nitrogen ratio | 3.298 |
| Organic matter | 11.158 |
| Moisture content | 77.767 |
| Ashes | 11.165 |
| Treatment Code | Description-Composition |
|---|---|
| PhoEnz | Enzymatic cocktail with phytases and alkaline, acid and neutral phosphatases on PDB |
| P. aeruginosa | P. aeruginosa ATC15442 as phosphate-solubilizing bacteria on PDB |
| Phy | Phytases on PHY |
| PhoEnz + P. aeruginosa | Combination of 5% and 5% |
| PhoEnz + P. aeruginosa + Phy | Combination of 3.3%, 3.3% and 3.3% |
| W | Deinoized water (control group) |
| Enzyme Type | Average Enzymatic Activity (UmgProtein) |
|---|---|
| Alkaline phosphatase | 0.242 ± 0.0086 |
| Acid phosphatase | 0.259 ± 0.0098 |
| Neutral phosphatase | 0.161 ± 0.0072 |
| Phytase | 0.005 ± 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Torres, A.E.; Rico-García, E.; Guzmán-Cruz, R.; Torres-Pacheco, I.; Tovar-Pérez, E.G.; Guevara-González, R.G. Addition of Phosphatases and Phytases to Mature Compost to Increase Available Phosphorus: A Short Study. Agronomy 2021, 11, 2555. https://doi.org/10.3390/agronomy11122555
Ortega-Torres AE, Rico-García E, Guzmán-Cruz R, Torres-Pacheco I, Tovar-Pérez EG, Guevara-González RG. Addition of Phosphatases and Phytases to Mature Compost to Increase Available Phosphorus: A Short Study. Agronomy. 2021; 11(12):2555. https://doi.org/10.3390/agronomy11122555
Chicago/Turabian StyleOrtega-Torres, Adrian Esteban, Enrique Rico-García, Rosario Guzmán-Cruz, Irineo Torres-Pacheco, Erik Gustavo Tovar-Pérez, and Ramón Gerardo Guevara-González. 2021. "Addition of Phosphatases and Phytases to Mature Compost to Increase Available Phosphorus: A Short Study" Agronomy 11, no. 12: 2555. https://doi.org/10.3390/agronomy11122555