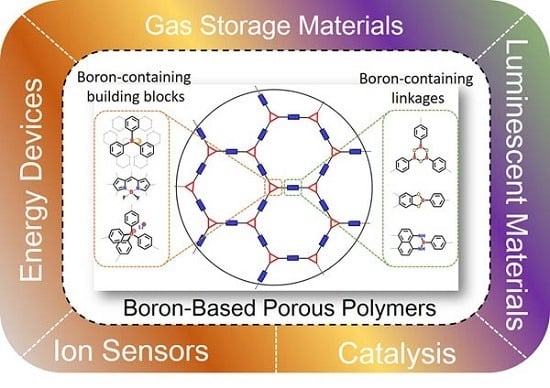
Recent Advances in Boron-Containing Conjugated Porous Polymers
Abstract
:1. Introduction
2. Networks with Different Boron-Based Building Blocks
2.1. Triarylborane-Based Building Blocks
2.2. Triphenyl Borate-Based Building Blocks
2.3. BODIPY-Based Building Blocks
2.4. Tetraphenylborate-Based Building Blocks
3. Networks with Different Boron-Based Linkers
3.1. B-O Type Linkers
3.2. B-N-Type Linkers
4. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.H. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Moler, D.B.; Li, H.L.; Chen, B.L.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortes, J.L.; Cote, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, M.T.; Du Prez, F.E. Porous polymer particles-A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.D.; Tan, B.; Trewin, A.; Su, F.; Rosseinsky, M.J.; Bradshaw, D.; Sun, Y.; Zhou, L.; Cooper, A.I. Microporous organic polymers for methane storage. Adv. Mater. 2008, 20, 1916–1921. [Google Scholar] [CrossRef]
- Han, S.S.; Furukawa, H.; Yaghi, O.M.; Goddard, W.A. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 2008, 130, 11580–11581. [Google Scholar] [CrossRef] [PubMed]
- Germain, J.; Fréchet, J.M.J.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-T.; Chen, Z.; Sun, J.; Huang, Z.-T.; Zheng, Q.-Y. Cyclotricatechylene based porous crystalline material: Synthesis and applications in gas storage. J. Mater. Chem. 2012, 22, 5369–5373. [Google Scholar] [CrossRef]
- Liu, Q. Monodisperse polystyrene nanospheres with ultrahigh surface area: Application for hydrogen storage. Macromol. Chem. Phys. 2010, 211, 1012–1017. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Snurr, R.Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Pulko, I.; Wall, J.; Krajnc, P.; Cameron, N.R. Ultra-high surface area functional porous polymers by emulsion templating and hypercrosslinking: Efficient nucleophilic catalyst supports. Chem. A Eur. J. 2010, 16, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F.-S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ying, J.Y. Main-chain organic frameworks with advanced catalytic functionalities. ACS Catal. 2015, 5, 2681–2691. [Google Scholar] [CrossRef]
- Pu, H.; Wang, D.; Yang, Z. Towards high water retention of proton exchange membranes at elevated temperature via hollow nanospheres. J. Membr. Sci. 2010, 360, 123–129. [Google Scholar] [CrossRef]
- Yang, J.S.; Swager, T.M. Fluorescent porous polymer films as TNT chemosensors: Electronic and structural effects. J. Am. Chem. Soc. 1998, 120, 11864–11873. [Google Scholar] [CrossRef]
- Lee, W.-E.; Lee, C.-L.; Sakaguchi, T.; Fujiki, M.; Kwak, G. Fluorescent viscosity sensor film of molecular-scale porous polymer with intramolecular π-stack structure. Macromolecules 2011, 44, 432–436. [Google Scholar] [CrossRef]
- Novotney, J.L.; Dichtel, W.R. Conjugated porous polymers For TNT vapor detection. ACS Macro Lett. 2013, 2, 423–426. [Google Scholar] [CrossRef]
- Yuan, K.; Guo-Wang, P.; Hu, T.; Shi, L.; Zeng, R.; Forster, M.; Pichler, T.; Chen, Y.; Scherf, U. Nanofibrous and graphene-templated conjugated microporous polymer materials for flexible chemosensors and supercapacitors. Chem. Mater. 2015, 27, 7403–7411. [Google Scholar] [CrossRef]
- Suresh, V.M.; Bandyopadhyay, A.; Roy, S.; Pati, S.K.; Maji, T.K. Highly luminescent microporous organic polymer with lewis acidic boron sites on the pore surface: Ratiometric sensing and capture of F(-) ions. Chem. A Eur. J. 2015, 21, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhou, Y.; Yan, D. Preparation of robust poly(ε-caprolactone) hollow spheres with controlled biodegradability. Macromol. Rapid Commun. 2006, 27, 1265–1270. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, Z.; Su, H.; Jing, X.; Sun, F.; Zhu, G. Targeted synthesis of a 2D ordered porous organic framework for drug release. Chem. Commun. 2011, 47, 6389–6391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, H.; Lee, J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Lee, K.C.; Youn, Y.S. Highly porous large poly(lactic-co-glycolic acid) microspheres adsorbed with palmityl-acylated exendin-4 as a long-acting inhalation system for treating diabetes. Biomaterials 2011, 32, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Eghtedari, M.; Motamedi, M.; Kotov, N.A. Inverted-colloidal-crystal hydrogel matrices as three-dimensional cell scaffolds. Adv. Func. Mater. 2005, 15, 725–731. [Google Scholar] [CrossRef]
- Kimmins, S.D.; Cameron, N.R. Functional porous polymers by emulsion templating: recent advances. Adv. Func. Mater. 2011, 21, 211–225. [Google Scholar] [CrossRef]
- Barbosa, E.F.; Silva, L.P. Nanoscale characterization of synthetic polymeric porous membranes: Scrutinizing their stiffness, roughness, and chemical composition. J. Membr. Sci. 2012, 407–408, 128–135. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, F.; Wu, D.; Forler, N.; Liang, H.; Wagner, M.; Gehrig, D.; Hansen, M.R.; Laquai, F.; Feng, X. Two-dimensional sandwich-type, graphene-based conjugated microporous polymers. Angew. Chem. Int. Ed. 2013, 52, 9668–9672. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Zhuang, X.; Bruller, S.; Feng, X.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, F.; Wu, D.; Feng, X. Graphene coupled schiff-base porous polymers: Towards nitrogen-enriched porous carbon nanosheets with ultrahigh electrochemical capacity. Adv. Mater. 2014, 26, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Chen, L.; Liu, J.; Parvez, K.; Liang, H.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.; Müllen, K. High-Performance electrocatalysts for oxygen reduction derived from cobalt porphyrin-based conjugated mesoporous polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Nickerl, G.; Wisser, F.M.; Nishio-Hamane, D.; Hosono, E.; Zhou, H.; Kaskel, S.; Eckert, J. An energy storage principle using bipolar porous polymeric frameworks. Angew. Chem. Int. Ed. 2012, 51, 7850–7854. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S.; Qi, X.; Wei, Z. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5414–5420. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Luo, Y.L.; Tan, B.E. Recent development of hypercrosslinked microporous organic polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.X.; Hou, Z.S.; Yao, Z.Q.; Zhuang, X.D.; Zhang, F.; Feng, X.L. Hypercrosslinked porous polymer nanosheets: 2D RAFT agent directed emulsion polymerization for multifunctional applications. Polym. Chem. 2015, 6, 7171–7178. [Google Scholar] [CrossRef]
- Weng, X.L.; Baez, J.E.; Khiterer, M.; Hoe, M.Y.; Bao, Z.B.; Shea, K.J. Chiral polymers of intrinsic microporosity: selective membrane permeation of enantiomers. Angew. Chem. Int. Ed. 2015, 54, 11214–11218. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Gehrig, D.; Forler, N.; Liang, H.; Wagner, M.; Hansen, M.R.; Laquai, F.; Zhang, F.; Feng, X. Conjugated microporous polymers with dimensionality-controlled heterostructures for green energy devices. Adv. Mater. 2015, 27, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Ren, H.; Ma, S.Q.; Cao, D.P.; Lan, J.H.; Jing, X.F.; Wang, W.C.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed.. 2009, 48, 9457–9460. [Google Scholar] [CrossRef] [PubMed]
- Ben, T.; Qiu, S.L. Porous aromatic frameworks: Synthesis, structure and functions. Crystengcomm 2013, 15, 17–26. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, F.; Li, L.; Cui, P.; Zhu, G. Porous aromatic frameworks with anion-templated pore apertures serving as polymeric sieves. Nat. Commun. 2014, 5, 4260. [Google Scholar] [CrossRef] [PubMed]
- Cao, C. a.; Zhuang, X.; Su, Y.; Zhang, Y.; Zhang, F.; Wu, D.; Feng, X. 2D polyacrylonitrile brush derived nitrogen-doped carbon nanosheets for high-performance electrocatalysts in oxygen reduction reaction. Polym. Chem. 2014, 5, 2057–2064. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhao, W.; Zhang, F.; Cao, Y.; Liu, F.; Bi, S.; Feng, X. A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton. Polym. Chem. 2016. [Google Scholar] [CrossRef]
- Schwab, M.G.; Fassbender, B.; Spiess, H.W.; Thomas, A.; Feng, X.; Mullen, K. Catalyst-free preparation of melamine-based microporous polymer networks through Schiff base chemistry. J. Am. Chem. Soc. 2009, 131, 7216–7217. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef] [PubMed]
- Vilela, F.; Zhang, K.; Antonietti, M. Conjugated porous polymers for energy applications. Energy Environ. Sci. 2012, 5, 7819. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Li, S. Novel functional organic network containing quaternary phosphonium and tertiary phosphorus. Macromolecules 2012, 45, 2981–2988. [Google Scholar] [CrossRef]
- Zhao, W.; Zhuang, X.; Wu, D.; Zhang, F.; Gehrig, D.; Laquai, F.; Feng, X. Boron-π-nitrogen-based conjugated porous polymers with multi-functions. J. Mater. Chem. A 2013, 1, 13878. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, H.; A, S.; Xia, H.; Mu, Y. Triarylboron-based fluorescent conjugated microporous polymers. RSC Adv. 2013, 3, 21267. [Google Scholar] [CrossRef]
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated microporous poly(aryleneethynylene) networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef] [PubMed]
- Neti, V.S.P.K.; Wang, J.; Deng, S.; Echegoyen, L. High and selective CO2 adsorption by a phthalocyanine nanoporous polymer. J. Mater. Chem. A 2015, 3, 10284–10288. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Wang, R. Tailorable synthesis of porous organic polymers decorating ultrafine palladium nanoparticles for hydrogenation of olefins. ACS Catal. 2015, 5, 948–955. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Wang, J.; Wang, R. Facile fabrication of ultrafine palladium nanoparticles with size- and location-control in click-based porous organic polymers. ACS Nano 2014, 8, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Zhou, T.Y.; Zhao, Q.L.; Fu, J.; Ma, Z.; Zhao, X. A triptycene-based microporous organic polymer bearing tridentate ligands and its application in Suzuki-Miyaura cross-coupling reaction. Macromol. Rapid Commun. 2015, 36, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.J.; Tranchemontagne, D.J.; Glover, T.G.; Hunt, J.R.; Yaghi, O.M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2010, 2, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, B.; Chai, S.-H.; Liu, H.; Dai, S. Hyper-crosslinked β-cyclodextrin porous polymer: An adsorption-facilitated molecular catalyst support for transformation of water-soluble aromatic molecules. Chem. Sci. 2016, 7, 905–909. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, X.; Su, Y.; Zhang, F.; Feng, X. Polyaniline nanosheet derived B/N co-doped carbon nanosheets as efficient metal-free catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7742–7746. [Google Scholar] [CrossRef]
- Luo, W.; Wang, B.; Heron, C.G.; Allen, M.J.; Morre, J.; Maier, C.S.; Stickle, W.F.; Ji, X. Pyrolysis of cellulose under ammonia leads to nitrogen-doped nanoporous carbon generated through methane formation. Nano Lett. 2014, 14, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, C.; Jin, T.; Wang, J.; Mahurin, S.M.; Mei, W.; Xiong, Y.; Hu, J.; Feng, X.; Liu, H.; et al. Thiazolothiazole-linked porous organic polymers. Chem. Commun. 2014, 50, 15055–15058. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Huang, N.; Chen, Y.; Zhang, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. Porous organic polymer films with tunable work functions and selective hole and electron flows for energy conversions. Angew. Chem. Int. Ed. 2016, 55, 3049–3053. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhuang, X.; Huang, Y.; Jiang, J.; Tian, H.; Wu, D.; Zhang, F.; Mai, Y.; Feng, X. Nitrogen-enriched hierarchically porous carbon materials fabricated by graphene aerogel templated Schiff-base chemistry for high performance electrochemical capacitors. Polym. Chem. 2015, 6, 1088–1095. [Google Scholar] [CrossRef]
- Ren, S.; Dawson, R.; Laybourn, A.; Jiang, J.-X.; Khimyak, Y.; Adams, D.J.; Cooper, A.I. Functional conjugated microporous polymers: From 1,3,5-benzene to 1,3,5-triazine. Polym. Chem. 2012, 3, 928–934. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Liu, P.; Wu, D.; Zhuang, X.; Zhang, F.; Feng, X. Compact coupled graphene and porous polyaryltriazine-derived frameworks as high performance cathodes for lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Bildirir, H.; Paraknowitsch, J.P.; Thomas, A. A tetrathiafulvalene (TTF)-conjugated microporous polymer network. Chem. A Eur. J. 2014, 20, 9543–9548. [Google Scholar] [CrossRef] [PubMed]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-based microporous polymer networks via chemical or electrochemical oxidative coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef]
- Bildirir, H.; Osken, I.; Ozturk, T.; Thomas, A. Reversible doping of a dithienothiophene-based conjugated microporous polymer. Chem. A Eur. J. 2015, 21, 9306–9311. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Schimanowitz, A.; Dawson, R.; Senkovska, I.; Kaskel, S.; Thomas, A. Cationic microporous polymer networks by polymerisation of weakly coordinating cations with CO2-storage ability. J. Mater. Chem. A 2014, 2, 11825–11829. [Google Scholar] [CrossRef]
- Qiao, S.; Huang, W.; Du, Z.; Chen, X.; Shieh, F.-K.; Yang, R. Phosphine oxide-based conjugated microporous polymers with excellent CO2 capture properties. New J. Chem. 2015, 39, 136–141. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, W.; Wang, W.; Zhang, F.; Zhuang, X.; Han, S.; Feng, X. One-pot approach to Pd-loaded porous polymers with properties tunable by the oxidation state of the phosphorus core. Polym. Chem. 2015, 6, 6351–6357. [Google Scholar] [CrossRef]
- Han, S.; Feng, Y.; Zhang, F.; Yang, C.; Yao, Z.; Zhao, W.; Qiu, F.; Yang, L.; Yao, Y.; Zhuang, X.; et al. Metal-phosphide-containing porous carbons derived from an ionic-polymer framework and applied as highly efficient electrochemical catalysts for water splitting. Adv. Func. Mater. 2015, 25, 3899–3906. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Xia, H.; Ding, X.; Luo, X.; Liu, X.; Mu, Y. Triarylboron-linked conjugated microporous polymers: sensing and removal of fluoride ions. Chem. A Eur. J. 2015, 21, 17355–17362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Han, S.; Zhuang, X.; Zhang, F.; Mai, Y.; Feng, X. Cross-linked polymer-derived B/N co-doped carbon materials with selective capture of CO2. J. Mater. Chem. A 2015, 3, 23352–23359. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Choi, T.J.; Park, H.W.; Chang, J.Y. Preparation of a microporous organic polymer by the thiol-yne addition reaction and formation of Au nanoparticles inside the polymer. Chem. Commun. 2015, 51, 9805–9808. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Wang, S. Impact of donor-acceptor geometry and metal chelation on photophysical properties and applications of triarylboranes. Acc. Chem. Res. 2009, 42, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Jäkle, F. Advances in the synthesis of organoborane polymers for optical, electronic, and sensory applications. Chem. Rev. 2010, 110, 3985–4022. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Stimuli and shape responsive ‘boron-containing’ luminescent organic materials. J. Mater. Chem. C 2016, 4, 2647–2662. [Google Scholar] [CrossRef]
- Wade, C.R.; Broomsgrove, A.E.; Aldridge, S.; Gabbai, F.P. Fluoride ion complexation and sensing using organoboron compounds. Chem. Rev. 2010, 110, 3958–3984. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Ham, J. Preparation of potassium alkynylaryltrifluoroborates from haloaryltrifluoroborates via sonogashira coupling reaction. Org. Lett. 2010, 12, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Liras, M.; Iglesias, M.; Sánchez, F. Conjugated microporous polymers incorporating BODIPY moieties as light-emitting materials and recyclable visible-light photocatalysts. Macromolecules 2016, 49, 1666–1673. [Google Scholar] [CrossRef]
- Fischer, S.; Schmidt, J.; Strauch, P.; Thomas, A. An anionic microporous polymer network prepared by the polymerization of weakly coordinating anions. Angew. Chem. Int. Ed. 2013, 52, 12174–12178. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yuan, Y.; Tian, Y.; Zhang, D.; Zhu, G. Highly efficient enrichment of volatile iodine by charged porous aromatic frameworks with three sorption sites. Angew. Chem. Int. Ed. 2015, 54, 12733–12737. [Google Scholar] [CrossRef] [PubMed]
- Van Humbeck, J.F.; Aubrey, M.L.; Alsbaiee, A.; Ameloot, R.; Coates, G.W.; Dichtel, W.R.; Long, J.R. Tetraarylborate polymer networks as single-ion conducting solid electrolytes. Chem. Sci. 2015, 6, 5499–5505. [Google Scholar] [CrossRef]
- Malek, N.; Maris, T.; Simard, M.; Wuest, J.D. Molecular tectonics. Selective exchange of cations in porous anionic hydrogen-bonded networks built from derivatives of tetraphenylborate. J. Am. Chem. Soc. 2005, 127, 5910–5916. [Google Scholar] [CrossRef] [PubMed]
- Blight, B.A.; Guillet-Nicolas, R.; Kleitz, F.; Wang, R.-Y.; Wang, S. Luminescent triarylboron-functionalized zinc carboxylate metal–organic framework. Inorg. Chem. 2013, 52, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Proń, A.; Baumgarten, M.; Müllen, K. Phenylene bridged boron−Nitrogen containing dendrimers. Org. Lett. 2010, 12, 4236–4239. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Eickhoff, D.; Marder, T.B.; Fox, M.A.; Low, P.J.; Dwyer, A.D.; Tozer, D.J.; Schwedler, S.; Brockhinke, A.; Stammler, H.-G.; et al. Experimental and theoretical studies on organic D-π-A systems containing three-coordinate boron moieties as both π-donor and π-acceptor. Chem. A Eur. J. 2012, 18, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lalancette, R.A.; Jäkle, F. π-expanded borazine: An ambipolar conjugated B–π–N macrocycle. Angew. Chem. Int. Ed. 2012, 51, 7994–7998. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.-R.; Liu, X.-Y.; Wang, S. Charge-transfer emission involving three-coordinate organoboron: V-Shape versus U-shape and impact of the spacer on dual emission and fluorescent sensing. Chem. A Eur. J. 2007, 13, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hillesheim, P.C.; Mahurin, S.M.; Wang, C.; Tian, C.; Brown, S.; Luo, H.; Veith, G.M.; Han, K.S.; Hagaman, E.W.; et al. Efficient CO2 capture by porous, nitrogen-doped carbonaceous adsorbents derived from task-specific ionic liquids. ChemSusChem 2012, 5, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Steeger, M.; Lambert, C. Charge-transfer interactions in tris-donor–tris-acceptor hexaarylbenzene redox chromophores. Chem. A Eur. J. 2012, 18, 11937–11948. [Google Scholar] [CrossRef] [PubMed]
- Parab, K.; Venkatasubbaiah, K.; Jäkle, F. Luminescent triarylborane-functionalized polystyrene: Synthesis, photophysical characterization, and anion-binding studies. J. Am. Chem. Soc. 2006, 128, 12879–12885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhuang, X.; Zhang, F.; Yang, L.; Bi, S.; Wu, D.; Yao, Y.; Graf, R.; Hansen, M.; Feng, X. From Lewis acid-based neutral porous polymers to anionic porous polymers. Chem. Sci. (under review).
- Entwistle, C.D.; Marder, T.B. Applications of three-coordinate organoboron compounds and polymers in optoelectronics. Chem. Mater. 2004, 16, 4574–4585. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Zhang, F.; Tang, R.; Fu, Y.; Wang, X.; Han, S.; Zhuang, X.; Feng, X. Triple boron-cored chromophores bearing discotic 5,11,17-triazatrinaphthylene-based ligands. Org. Lett. 2016, 18, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, Y.; Ding, J.; Liu, L.; Qin, J.; Zhao, Y.; Hou, H.; Fan, Y. Spectroscopic and crystallographic investigations of novel BODIPY-derived metal–organic frameworks. Inorg. Chem. 2015, 54, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-harvesting metal–organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yang, X.; Stern, C.L.; Marks, T.J. Cationic metallocene polymerization catalysts based on tetrakis(pentafluorophenyl)borate and its derivatives. probing the limits of anion “noncoordination” via a synthetic, solution dynamic, structural, and catalytic olefin polymerization study. Organometallics 1997, 16, 842–857. [Google Scholar] [CrossRef]
- Jutzi, P.; Müller, C.; Stammler, A.; Stammler, H.-G. Synthesis, crystal structure, and application of the oxonium acid [H(OEt2)2]+[B(C6F5)4]−. Organometallics 2000, 19, 1442–1444. [Google Scholar] [CrossRef]
- Türp, D.; Wagner, M.; Enkelmann, V.; Müllen, K. Synthesis of nanometer-sized, rigid, and hydrophobic anions. Angew. Chem. Int. Ed. 2011, 50, 4962–4965. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Bonder, E.M.; Jäkle, F. Weakly coordinating amphiphilic organoborate block copolymers. J. Am. Chem. Soc. 2010, 132, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Nishiyabu, R.; James, T.D. Hierarchical supramolecules and organization using boronic acid building blocks. Chem. Commun. 2015, 51, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Marcon, V.; Pisula, W.; Hansen, M.R.; Kirkpatrick, J.; Grozema, F.; Andrienko, D.; Kremer, K.; Mullen, K. Towards high charge-carrier mobilities by rational design of the shape and periphery of discotics. Nat. Mater. 2009, 8, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 2008, 47, 8826–8830. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A photoconductive covalent organic framework: Self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 2009, 48, 5439–5442. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, L.; Honsho, Y.; Saeki, A.; Seki, S.; Irle, S.; Dong, Y.; Nagai, A.; Jiang, D. High-rate charge-carrier transport in porphyrin covalent organic frameworks: Switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 2012, 51, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ding, X.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S.; et al. Charge dynamics in a donor–acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 2013, 52, 2017–2021. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Burress, J.W.; Ford, J.; Yildirim, T. Porous graphene oxide frameworks: Synthesis and gas sorption properties. J. Mater. Chem. 2011, 21, 11323–11329. [Google Scholar] [CrossRef]
- Kahveci, Z. Synthesis and characterization of multifunctional porous diazaborole-linked polymers. In Proceedings of the Abstracts of Papers, 248th ACS National Meeting&Exposition, San Francisco, CA, USA, 10–14 August 2014.











© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Zhao, W.; Han, S.; Zhuang, X.; Lin, H.; Zhang, F. Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers 2016, 8, 191. https://doi.org/10.3390/polym8050191
Qiu F, Zhao W, Han S, Zhuang X, Lin H, Zhang F. Recent Advances in Boron-Containing Conjugated Porous Polymers. Polymers. 2016; 8(5):191. https://doi.org/10.3390/polym8050191
Chicago/Turabian StyleQiu, Feng, Wuxue Zhao, Sheng Han, Xiaodong Zhuang, Hualin Lin, and Fan Zhang. 2016. "Recent Advances in Boron-Containing Conjugated Porous Polymers" Polymers 8, no. 5: 191. https://doi.org/10.3390/polym8050191