Enhanced Water Vapor Transmission through Porous Membranes Based on Melt Blending of Polystyrene Sulfonate with Polyethylene Copolymers and Their CNT Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Copolymer through Free Radical Polymerization
2.3. Surface Modification of Carbon Nanotubes
2.3.1. Surface Polymerization of SSNa Monomer onto Carbon Nanotubes
2.3.2. Functionalization of Carbon Nanotubes with Undecyl (C11-) Radicals
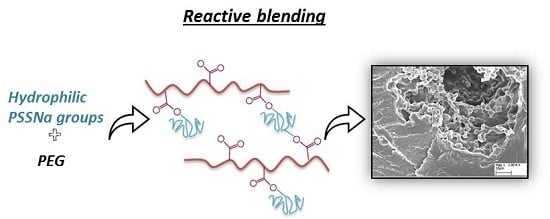
2.4. Reactive Blending and Membrane Preparation
2.5. Characterization Techniques
2.6. Water Vapor Transmission Rate
3. Results and Discussion
3.1. Surface Modification of Carbon Nanotubes
3.2. Chemical Structure of Membranes
3.3. Morphology of the Blends
3.4. TGA Characterization
3.5. Water Vapor Transmission
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sereewatthanawut, I.; Boam, A.T.; Livingston, A.G. Polymeric membrane nanofiltration and its application to separations in the chemical industries. Macromol. Symp. 2008, 264, 184–188. [Google Scholar] [CrossRef]
- Mondal, S.; Cassano, A.; Tasselli, F.; De, S. A generalized model for clarification of fruit juice during ultrafiltration under total recycle and batch mode. J. Membr. Sci. 2011, 366, 295–303. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Jegal, J.; Lee, K.H. Optical resolution of α-amino acids through enantioselective polymeric membranes based on polysaccharides. J. Membr. Sci. 2003, 213, 273–283. [Google Scholar] [CrossRef]
- Taylor, J.S.; Jacobs, E.P. Reverse Osmosis and Nanofiltration. In Water Treatment Membrane Processes; Mallevialle, J., Odendaal, P.E., Wiesner, M.R., Eds.; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Tan, K.; Obendorf, S.K. Surface modification of microporous polyurethane membrane with poly(ethylene glycol) to develop a novel membrane. J. Membr. Sci. 2006, 274, 150–158. [Google Scholar] [CrossRef]
- Nie, F.Q.; Xu, Z.K.; Yang, Q.; Wu, J.; Wan, L.S. Surface modification of poly(acryloniterrile-co-maleic acid) membranes by the immobilization of poly(ethylene glycol). J. Membr. Sci. 2004, 235, 147–155. [Google Scholar] [CrossRef]
- Wu, P.C.; Jones, G.; Shelley, C.; Woelfli, B. Novel microporous films and their composites. J. Eng. Fiber. Fabr. 2007, 2, 49–59. [Google Scholar]
- Ciardelli, F.; Penczek, S. (Eds.) Modification and Blending of Synthetic and Natural Macromolecules; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 155–199.
- Koulouri, E.G.; Georgaki, A.X.; Kallitsis, J.K. Reactive compatibilization of aliphatic polyamides with functionalized polyethylenes. Polymer 1997, 38, 4185–4192. [Google Scholar] [CrossRef]
- Gravalos, K.G.; Kallitsis, J.K.; Kalfoglou, N.K. In situ compatibilization of poly(ethylene terephthaIate)/poly(ethylene-co-ethyl acrylate) blends. Polymer 1995, 36, 1393–1399. [Google Scholar] [CrossRef]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity trade off in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Vankelecom, I.F.J.; Merckx, E.; Luts, M.; Uytterhoeven, J.B. Incorporation of zeolites in polyimide membranes. J. Phys. Chem. 1995, 99, 13187–13192. [Google Scholar] [CrossRef]
- Ballinas, L.; Torras, C.; Fierro, V.; Garcia-Valls, R. Factors influencing activated carbon polymeric composite membrane structure and performance. J. Phys. Chem. Sol. 2004, 65, 633–637. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Multi-walled carbon nanotubes (MWNTs)/polysulfone (PSU) mixed matrix hollow fiber membranes for enhanced water treatment. J. Membr. Sci. 2013, 437, 237–248. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Li, S.; Liao, G.; Liu, Z.; Pan, Y.; Wu, Q.; Weng, Y.; Zhang, X.; Yang, Z.; Tsui, O.K.C. Enhanced water flux in vertically aligned carbon nanotube arrays and polyethersulfone composite membranes. J. Mater. Chem. A 2014, 2, 12171–12176. [Google Scholar] [CrossRef]
- Ismail, A.F.; Goh, P.S.; Sanip, S.M.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Choi, J.H.; Legal, J.; Kim, W.N. Modification of performances of various membranes using MWCNTs as a modifier. Macromol. Symp. 2007, 249–250, 610–617. [Google Scholar] [CrossRef]
- Mondal, S.; Hu, J.L. Microstructure and water vapor transport properties of functionalized carbon nanotube-reinforced dense segmented polyurethane composite membranes. Polym. Eng. Sci. 2008, 48, 1718–1724. [Google Scholar] [CrossRef]
- Mondal, D.; Bhowmick, B.; Mollick, M.M.R.; Maity, D.; Mukhopadhyay, A.; Rana, D.; Chattopadhyay, D. Effect of clay concentration on morphology and properties of hydroxypropylmethyl cellulose films. Carbohydr. Polym. 2013, 96, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Mollick, M.M.R.; Bhowmick, B.; Maity, D.; Bain, M.K.; Rana, D.; Mukhopadhyay, A.; Dana, K.; Chattopadhyay, D. Effect of poly(vinyl pyrrolidone) on the morphology and physical properties of poly(vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog. Nat. Sci. Mater. Int. 2013, 23, 579–587. [Google Scholar] [CrossRef]
- Saha, N.R.; Sarkar, G.; Roy, I.; Rana, D.; Bhattacharyya, A.; Adhikari, A.; Mukhopadhyay, A.; Chattopadhyay, D. Synthesis and characterization of methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr. Polym. 2016, 136, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Bhowmick, B.; Mollick, Md.M.R.; Maity, D.; Saha, N.R.; Rangarajan, V.; Rana, D.; Sen, R.; Chattopadhyay, D. Antimicrobial activity and biodegradation behaviour of poly(butylene adipate-co-butylene terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40079. [Google Scholar] [CrossRef]
- Hale, W.R.; Dohrer, K.K.; Tant, M.R.; Sand, I.D. A diffusion model for water vapor transmission through microporous polyethylene /CaCO3 films. Coll. Surf. A Physiochem. Eng. Asp. 2001, 187–188, 483–491. [Google Scholar] [CrossRef]
- Hudson, J.L.; Casavant, M.J.; Tour, J.M. Water-soluble, exfoliated, nonroping single-wall carbon nanotubes. J. Am. Chem. Soc. 2004, 126, 11158–11159. [Google Scholar] [CrossRef] [PubMed]
- Usrey, M.L.; Lippmann, E.S.; Strano, M.S. Evidence for a two-step mechanism in electronically selective single-walled carbon nanotube reactions. J. Am. Chem. Soc. 2005, 127, 16129–16135. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Gao, C.; Yan, D. Functionalization of multiwalled carbon nanotubes by atom transfer radical polymerization and defunctionalization of the products. Macromolecules 2004, 37, 4022–4030. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Lainioti, G.C.; Gialeli, C.; Barbouri, D.; Kouravelou, K.B.; Karamanos, N.K.; Voyiatzis, G.A.; Kallitsis, J.K. Preparation and toxicological assessment of functionalized carbon nanotube-polymer hybrids. PLoS ONE 2014, 9, e107029. [Google Scholar] [CrossRef] [PubMed]
- Koval’chuk, A.A.; Shevchenko, V.G.; Shchegolikhin, A.N.; Nedorezova, P.M.; Klyamkina, A.N.; Aladyshev, A.M. Effect of Carbon nanotube functionalization on the structural and mechanical properties of polypropylene/MWCNT composites. Macromolecules 2008, 41, 7536–7542. [Google Scholar] [CrossRef]
- Pire, M.; Norvez, S.; Iliopoulos, I.; Rossignol, B.; Leibler, L. Imidazole-promoted acceleration of crosslinking in epoxidized natural rubber/dicarboxylic acid blends. Polymer 2011, 52, 5243–5249. [Google Scholar] [CrossRef]
- Standard test methods for water vapor transmission of materials, ASTM E96/E96M-10. Available online: http://www.astm.org/Standards/E96.htm (accessed on 10 April 2016).
- Andrikopoulos, K.S.; Bounos, G.; Tasis, D.; Sygellou, L.; Drakopoulos, V.; Voyiatzis, G.A. The effect of thermal reduction on the water vapor permeation in graphene oxide membranes. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wei, F. A treatment method to give separated multi-walled carbon nanotubes with high purity, high crystallization and a large aspect ratio. Carbon 2003, 41, 2939–2948. [Google Scholar] [CrossRef]
- Guo, G.; Yang, D.; Wang, C.; Yang, S. Fishing polymer brushes on single-walled carbon nanotubes by in-situ free radical polymerization in a poor solvent. Macromolecules 2006, 39, 9035–9040. [Google Scholar] [CrossRef]
- Ko, J.H.; Yoon, C.S.; Chang, J.H. Polypropylene nanocomposites with various functionalized-multiwalled nanotubes: Thermomechanical properties, morphology, gas permeation, and optical transparency. J. Polym. Sci. Part B 2011, 49, 244–254. [Google Scholar] [CrossRef]
- Merfeld, G.; Molaison, C.; Koeniger, R.; Acar, A.E.; Mordhorst, S.; Suriano, J.; Irwin, P.; Warner, R.S.; Gray, K.; Smith, M.; et al. Acid/epoxy reaction catalyst screening for low temperature (120 °C) powder coatings. Prog. Org. Coat. 2005, 52, 98–109. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Tech. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Hu, Y.; Topolkaraev, V.; Hiltner, A.; Baer, E. Measurement of water vapor transmission rate in highly permeable films. J. Appl. Polym. Sci. 2001, 81, 1624–1633. [Google Scholar] [CrossRef]
- Tock, R.W. Permeabilities and water vapor transmission rates for commercial polymer films. Adv. Polym. Tech. 1983, 3, 223–231. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Cunningham, R.E.; Williams, R.J.J. Diffusion in Gases and Porous Media; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Kalka, A.; Garde, S.; Hummer, G. Osmotic Water Transport through carbon nanotubes membranes. Proc. Natl. Acad. Sci. USA 2003, 100, 10175–10180. [Google Scholar]
- Yzeiri, I.; Patra, N.; Kràl, P. Porous carbon nanotubes: Molecular adsorption, transport and separation. J. Chem. Phys. 2014, 140, 104704. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Libera, J.A.; Guvenc-Yazicioglu, A.; Megaridis, C.M. In situ multiphase fluid experiments in hydrothermal carbon nanotubes. Appl. Phys. Lett. 2001, 79, 1021–1023. [Google Scholar] [CrossRef]
- Mao, Z.; Sinnot, S.B. A computational study of molecular diffusion and dynamic flow through carbon nanotubes. J. Phys. Chem. B 2000, 104, 4618–4624. [Google Scholar] [CrossRef]
- Holt, J.K.; Noy, A.; Huser, T.; Eaglesham, D.; Bakajin, O. Fabrication of a carbon Nanotube-embedded silicon nitride membrane for studies of nanometer-scale mass transport. Nano Lett. 2004, 4, 2245–2250. [Google Scholar] [CrossRef]
- Joseph, S.; Aluru, N. Why are carbon nanotubes fast transporters of water? Nano Lett. 2008, 8, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Whitby, M.; Quirke, N. Fluid flow in carbon nanotubes and nanopipes. Nat. Nanotechnol. 2007, 2, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Pascal, T.A.; Goddard, W.A.; Jung, Y. Entropy and the driving force for the filling of carbon nanotubes with water. Proc. Natl. Acad. Sci. USA 2011, 108, 11794–11798. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, L.C.; Grubb, D.T.; Meyers, G.F. Polymer Microscopy, 3rd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]









| Membrane code | Polymers | Composition (wt %) | Loss (%) |
|---|---|---|---|
| M1 | P(ethylene-co-AΑ0.28)/P(SSNa-co-GMA0.2)/PEG | 60/10/30 | 28 |
| M2 | P(ethylene-co-AΑ0.28)/P(SSNa-co-GMA0.2)/PEG | 50/10/40 | 20 |
| M3 | P(ethylene-co-AΑ0.28)/P(SSNa-co-GMA0.1)/PEG | 60/10/30 | 28 |
| M4 | P(ethylene-co-AΑ0.28)/P(SSNa-co-GMA0.1)/PEG | 50/10/40 | 40 |
| M5 | P(ethylene-co-AΑ0.28)/P(SSNa-co-GMA0.1)/PEG | 50/15/35 | 35 |
| Membrane code | Functionalized MWCNTs | Composition (wt %) | Loss (%) |
|---|---|---|---|
| M3-f-CNTs(PSSNa) | MWCNTs-g-PSSNa | 60/10/30 | 28 |
| M4-f-CNTs(PSSNa) | MWCNTs-g-PSSNa | 50/10/40 | 25 |
| M5-f-CNTs(PSSNa) | MWCNTs-g-PSSNa | 50/15/35 | 30 |
| M3-f-CNTs(C11) | MWCNTs-g-C11 | 60/10/30 | 28 |
| M4-f-CNTs(C11) | MWCNTs-g-C11 | 50/10/40 | 38 |
| M5-f-CNTs(C11) | MWCNTs-g-C11 | 50/15/35 | 33 |
| Sample description | Composition (wt %) | Thickness (μm) | Sp.WVTR (μm·g·m−2·min−1) |
|---|---|---|---|
| PE | Pure | 40 | 1.94 |
| Celgard 2400 | – | 25 | 42.88 |
| M3 | 60/10/30 | 70 | 11.18 |
| M5 * | 50/15/35 | 260 | 18.05 |
| M5-f-CNTs(C11) * | 49/35/15/1 | 150 | 53.13 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lainioti, G.C.; Bounos, G.; Voyiatzis, G.A.; Kallitsis, J.K. Enhanced Water Vapor Transmission through Porous Membranes Based on Melt Blending of Polystyrene Sulfonate with Polyethylene Copolymers and Their CNT Nanocomposites. Polymers 2016, 8, 190. https://doi.org/10.3390/polym8050190
Lainioti GC, Bounos G, Voyiatzis GA, Kallitsis JK. Enhanced Water Vapor Transmission through Porous Membranes Based on Melt Blending of Polystyrene Sulfonate with Polyethylene Copolymers and Their CNT Nanocomposites. Polymers. 2016; 8(5):190. https://doi.org/10.3390/polym8050190
Chicago/Turabian StyleLainioti, Georgia Ch., Giannis Bounos, George A. Voyiatzis, and Joannis K. Kallitsis. 2016. "Enhanced Water Vapor Transmission through Porous Membranes Based on Melt Blending of Polystyrene Sulfonate with Polyethylene Copolymers and Their CNT Nanocomposites" Polymers 8, no. 5: 190. https://doi.org/10.3390/polym8050190