Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials and Reagents
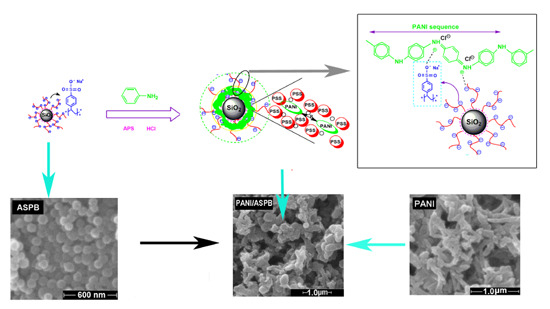
2.2. Preparation of PANI/ASPB Nanocomposite
2.3. Structural and Morphological Analysis
2.4. Performance Tests

3. Results and Discussion
3.1. Structural Analysis-FTIR Spectra

3.2. Structural Analysis—UV-VIS Spectra

3.3. Structural Analysis—XRD Patterns

3.4. Thermal Stability

3.5. Solubility

3.6. Evaluation of Dopants
| Properties | Samples | ||||
|---|---|---|---|---|---|
| PANI | PANI/ASPB | PANI/SiO2 | PANI/PSS | PANI/CSPB | |
| Electrical conductivity (S/cm) | 7.0 | 19.3 | 18.2 | 14.6 | 8 |
| Conductivity of saturated solution (μS/cm) | 3.78 | 8.4 | 5.8 | 7 | 6.7 |
| Exothermic peak (°C) | 268 | 294 | 280 | 270 | inconspicuous |
3.7. Electrical Conductivity and Its Influence Factors

3.7.1. Polymerization Temperature

3.7.2. The Amount of ASPB

3.7.3. Polymerization Time and Molecular Weight of Grafted Brushes

3.8. Doping Mechanism and Morphological Analysis—SEM Images
3.8.1. The Structure of Polyaniline

3.8.2. Three-Dimensional Variable Range Hopping (3D VRH) Theoretical Models

3.8.3. Template Theory
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sangoi, R.; Smith, C.G.; Seymour, M.D.; Venkataraman, J.N.; Clark, D.M.; Kleper, M.L.; Kahn, B.E. Printing radio frequency identification (RFID) tag antennas using inks containing metal nanoparticles. J. Disper. Sci. Technol. 2004, 25, 513. [Google Scholar] [CrossRef]
- Huang, Y.; Su, N.; Zhang, X.Z.; Zhao, J.J.; Li, H.B.; Liu, X.H.; Zhang, H.N. Controllable synthesis and characterization of poly(aniline-co-pyrrole) using anionic spherical polyelectrolyte brushes as dopant and template. Polym. Compos. 2014, 5, 1858–1863. [Google Scholar] [CrossRef]
- Miles, S.B. RFID Technology and Applications; Cambridge University Press: London, UK, 2011; pp. 6–8. [Google Scholar]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Berggren, M.; Nilsson, D.; Robinson, N.D. Organic materials for printed electronics. Nat. Mater. 2007, 6, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, J.; Haldolaarachchige, N.; Ryu, J.; Young, D.P.; Wei, S.; Guo, Z. Synthetic process engineered polyaniline nanostructures with tunable morphology and physical properties. Polymer 2012, 53, 2109–2120. [Google Scholar] [CrossRef]
- Karwa, A. Printing Studies with Conductive Inks and Exploration of New Conducting Polymer Compositions. Master’s Thesis, Rochester Institute of Technology, Rochester, New York, NY, USA, March 2006. [Google Scholar]
- Hara, K.; Kurashige, M.; Ito, S.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Novel polyene dyes for highly efficient dye-sensitized solar cells. Chem. Commun. 2003, 252–253. [Google Scholar] [CrossRef]
- Shiraknwa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Saurin, M.; Armes, S.P. Study of the chemical polymerization of pyrrole onto printed circuit boards for electroplating applications. J. Appl. Polym. Sci. 1995, 56, 41–50. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Xu, W. Investigating thermoelectric properties of doped polyaniline nanowires. Synth. Met. 2014, 189, 177–182. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Pei, W.B.; Huang, L.; Xu, W.; Zhang, Q. Polypyrrole nanotube film for flexible thermoelectric application. Synth. Met. 2014, 196, 173–177. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, J.; Kjelleberg, S.; Loo, J.S.C.; Zhang, Q. Employing a flexible and low-cost polypyrrole nanotube membrane as an anode to enhance current generation in microbial fuel cells. Small 2015, 11, 3440–3443. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huang, Y.; Zhang, X.; Wang, Q.; Zhu, J.; Shao, L.; Haldolaarachchige, N.; Young, D.P.; Wei, S.; Guo, Z. Magnetoresistive polyaniline-magnetite nanocomposites with negative dielectrical properties. Polymer 2012, 53, 801–809. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Wang, J.; Xu, P.; Han, X. Synthesis of polyaniline nanofibers with high electrical conductivity from CTAB-SDBS mixed surfactants. Mater. Lett. 2011, 65, 3601–3604. [Google Scholar] [CrossRef]
- Feng, X.M.; Liu, Y.G.; Lu, C.L.; Zhu, J.-J. One-step synthesis of AgCl/polyaniline core–shell composites with enhanced electroactivity. Nanotechnology 2006, 17, 3578–3583. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chakane, S.; Samui, A.B.; Krishnamurthy, V.N.; Bhoraskar, S.V. Humidity sensing with weak acid-doped polyaniline and its composites. Sens. Actuator B Chem. 2003, 96, 124–129. [Google Scholar] [CrossRef]
- Shambharkar, B.H.; Umare, S.S. Synthesis and characterization of polyaniline/NiO nanocomposite. J. Appl. Polym. Sci. 2011, 122, 1905–1912. [Google Scholar] [CrossRef]
- Vhanakhande, B.B.; Jadhav, S.V.; Kulkarni, D.C.; Puri, V. Investigations on the microwave properties of electropolymerised polyaniline thin film. Microw. Opt. Technol. Lett. 2008, 50, 761–766. [Google Scholar] [CrossRef]
- Lin, Q.Q.; Li, Y.; Yang, M.J. Polyaniline nanofiber humidity sensor prepared by electrospinning. Sens. Actuators B Chem. 2012, 161, 967–972. [Google Scholar] [CrossRef]
- Su, N.; Gu, P.; Zhao, J.J. Preparation and properties of polyaniline—Anionic sdherical polyelectrolyte brushes (PANI/ASPB) nanocomposites. Micro Nano Lett. 2015, 10, 175–178. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Zheng, H.M. Synthesis and characterization of poly(sodium-p-styrene sulfonate)/modified SiO2 spherical brushes. eXPRESS Polym. Lett. 2012, 6, 680–686. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Yuan, S.J.; Yi, S.P.; Yin, E.Q. Synthesis and characterization of polypyrrole doped with anionic spherical polyelectrolyte brushes. eXPRESS Polym. Lett. 2012, 6, 697–705. [Google Scholar]
- Kulkarni, M.V.; Viswanath, A.K.; Marimuthu, R.; Seth, T. Spectroscopic, transport, and morphological studies of polyaniline doped with inorganic acids. Polym. Eng. Sci. 2004, 44, 1676–1681. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M.; Janata, J. Acid doping of polyaniline: Spectroscopic and electrochemical studies. J. Phys. Chem. B 1999, 103, 10992–10998. [Google Scholar] [CrossRef]
- Mavundla, S.E.; Malgas, G.F.; Baker, P.; Iwuoha, E.I. Physicochemical and morphological properties of poly(aniline-co-pyrrole). Electroanalysis 2008, 20, 2347–2353. [Google Scholar] [CrossRef]
- Kuo, C.-T.; Chen, S.-A.; Hwang, G.-W.; Kuo, H.-H. Field-effect transistor with the water-soluble self-acid-doped polyaniline thin films as semiconductor. Synth. Met. 1998, 93, 155–160. [Google Scholar] [CrossRef]
- Souza, F.G.; Soares, B.G. Methodology for determination of PANI. DBSA content in conductive blends by using UV-vis spectrometry. Polym. Test. 2006, 25, 512–517. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, Z.; Wang, Z.; Zhang, F.; Wang, S. A novel strategy for the synthesis of polyaniline nanostructures with controlled morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Pouget, J.P.; Jozefowicz, M.E.; Epstein, A.J.; Tang, X.; MacDiarmid, A.G. X-ray structure of polyaniline. Macromolecules 1991, 24, 779–789. [Google Scholar] [CrossRef]
- Yin, W.; Ruckenstein, E. Soluble polyaniline co-doped with dodecyl benzene sulfonic acid and hydrochloric acid. Synth. Met. 2000, 108, 39–46. [Google Scholar] [CrossRef]
- Maeda, S.; Amres, S.P. Rreparatlon of novel polypyrrole-silica colloidal nanocomposites. J. Colloid Interface Sci. 1993, 159, 257–259. [Google Scholar] [CrossRef]
- Hu, X.Q.; Lu, Y.; Liu, J.H. Synthesis of polypyrrole microtubes with actinomorphic morphology in the presence of a beta-cyclodextrin derivative-methyl orange inclusion complex. Macro. Rapid Commun. 2004, 25, 1117–1120. [Google Scholar] [CrossRef]
- McManus, P.M.; Yang, S.C.; Cushman, R.J. Elecrtochemical doping of aniline: Eeffest on conductivity and optical spectra. J. Chem. Soc. Chem. Commun. 1985, 22, 1556–1557. [Google Scholar] [CrossRef]
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a novel conducting polymer: Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J. Chem. Soc. 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Stafström, S.; Brédas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; MacDiarmid, A.G. Polaron lattice in highly conducting polyaniline: Theoretical and optical studies. Phys. Rev. Lett. 1987, 59, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Grtega, M.M.; Inoue, M.B.; Inoue, M.J. Chemical synthesis of highly conducting polyrrole by the use of copper perchlorate as an oxidant. Synth. Met. 1989, 28, 65–70. [Google Scholar] [CrossRef]
- Gosh, M.; Barman, A.; Meikap, A.K.; De, S.K.; Chatterjee, S. Hopping transport in HCl doped conducting polyaniline. Phys. Lett. A 1999, 260, 138–148. [Google Scholar]
- Jaroslav, S.; Pavel, K.; Aubrey, D.J. The formation of polyaniline and the nature of its structures. Polymer 1996, 37, 367–369. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N. Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers 2015, 7, 1599-1616. https://doi.org/10.3390/polym7091473
Su N. Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers. 2015; 7(9):1599-1616. https://doi.org/10.3390/polym7091473
Chicago/Turabian StyleSu, Na. 2015. "Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property" Polymers 7, no. 9: 1599-1616. https://doi.org/10.3390/polym7091473