Size-Controlled Nanomicelles of Poly(lactic acid)–Poly(ethylene glycol) Copolymers with a Multiblock Configuration
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polycondensation
2.3. Preparation of Micelle Suspension
2.4. Measurement
3. Results
3.1. Synthesis of Multiblock Copolymers


| Sample name | Temperature (°C) | Reaction time (h) | Mn a (Da) | Mw a (Da) | Mw/Mn a | PLA/PEG b (w/w) | Multiblock index c |
|---|---|---|---|---|---|---|---|
| LE(m)-1.35 | 150 | 20 | 3,600 | 6,100 | 1.67 | 29.1:70.9 | 1.35 |
| LE(m)-1.53 | 150 | 35 | 4,200 | 6,900 | 1.66 | 29.1:70.9 | 1.53 |
| LE(m)-2.27 | 150 | 65 | 6,900 | 9,200 | 1.34 | 21.2:78.8 | 2.27 |
| LE(m)-1.38 | 180 | 15 | 4,300 | 7,000 | 1.62 | 37.1:62.9 | 1.38 |
| LE(m)-2.78 | 180 | 25 | 9,600 | 14,000 | 1.46 | 36.4:63.6 | 2.78 |
| LE(m)-2.75 | 180 | 35 | 8,300 | 13,400 | 1.63 | 34.3:65.7 | 2.75 |
| LE(m)-2.93 | 180 | 65 | 9,100 | 12,700 | 1.40 | 26.2:73.8 | 2.93 |



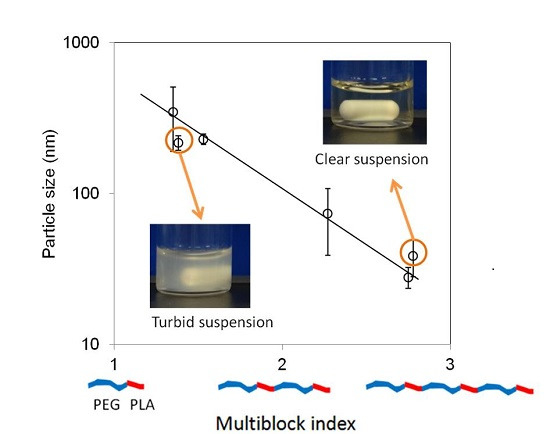
3.2. Micelles





4. Discussions
4.1. Racemization
4.2. Micelle Size
4.3. Crystallinity
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1995, 263, 1600–1603. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Fujiwara, T.; Mukose, T.; Yamaoka, T.; Yamane, H.; Sakurai, S.; Kimura, Y. Novel thermo-responsive formation of a hydrogel by stereo-complexation between PLLA–PEG–PLLA and PDLA–PEG–PDLA block copolymers. Macromol. Biosci. 2001, 1, 204–208. [Google Scholar] [CrossRef]
- Fujiwara, T.; Miyamoto, M.; Kimura, Y.; Iwata, T.; Doi, Y. Self-organization of diblock and triblock copolymers of poly(l-lactide) and poly(oxyethylene) into nanostructured bands and their network system. Proposition of a doubly twisted chain conformation of poly(l-lactide). Macromolecules 2001, 34, 4043–4050. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kimura, Y. Macromolecular organization of poly(l-lactide)-block-polyoxyethylene into bio-inspired nano-architectures. Macromol. Biosci. 2002, 2, 11–23. [Google Scholar] [CrossRef]
- Yue, J.; Liu, S.; Xie, Z.; Xing, Y.; Jing, X. Size-dependent biodistribution and antitumor efficacy of polymer micelle drug delivery systems. J. Mater. Chem. B 2013, 1, 4273–4280. [Google Scholar] [CrossRef]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994, 83, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Takahashi, Y.; Ohta, T.; Miyamoto, M.; Murakami, A.; Kimura, Y. Synthesis and properties of multiblock copolymers consisting of poly(l-lactic acid) and poly(oxypropylene-co-oxyethylene) prepared by direct polycondensation. J. Polym. Sci. A Polym. Chem. 1999, 37, 1513–1521. [Google Scholar] [CrossRef]
- Ehashi, T.; Kakinoki, S.; Yamaoka, T. Water absorbing and quick degradable PLLA/PEG multiblock copolymers reduce the encapsulation and inflammatory cytokine production. J. Artif. Organs 2014, 17, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Takahashi, Y.; Fujisato, T.; Kimura, Y. Preparation and evaluations of PLLA-based multiblock copolymer fibers as a novel suture. Jpn. J. Artif. Organs 2000, 29, 239–244. [Google Scholar]
- Yamaoka, T.; Njatawidjaja, E.; Kasai, A.; Agudelo, C.A.; Ehashi, T.; Kakinoki, S.; Kato, S.; Mahara, A. Elastic/adhesive double-layered PLA–PEG multiblock copolymer membranes for postoperative adhesion prevention. Polym. Degrad. Stabil. 2013, 98, 2168–2176. [Google Scholar] [CrossRef]
- Bae, Y.H.; Huh, K.M.; Kim, Y.; Park, K.H. Biodegradable amphiphilic multiblock copolymers and their implications for biomedical applications. J. Control. Release 2000, 64, 3–13. [Google Scholar] [CrossRef]
- Na, K.; Lee, K.H.; Lee, D.H.; Bae, Y.H. Biodegradable thermo-sensitive nanoparticles from poly(l-lactic acid)/poly(ethylene glycol) alternating multi-block copolymer for potential anti-cancer drug carrier. Eur. J. Pharm. Sci. 2006, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.I.; Lee, C.W.; Miyamoto, M.; Kimura, Y. Melt polycondensation of l-lactic acid with Sn(II) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight poly(l-lactic acid). J. Polym. Sci. A Polym. Chem. 2000, 38, 1673–1679. [Google Scholar] [CrossRef]
- Moon, S.I.; Taniguchi, I.; Miyamoto, M.; Kimura, Y.; Lee, C.W. Synthesis and properties of high-molecular-weight poly(l-lactic acid) by melt/solid polycondensation under different reaction conditions. High Perform Polym. 2001, 13, S189–S196. [Google Scholar] [CrossRef]
- Moon, S.I.; Deguchi, K.; Miyamoto, M.; Kimura, Y. Synthesis of polyglactin by melt/solid polycondensation of glycolic/l-lactic acids. Polym. Int. 2004, 53, 254–258. [Google Scholar] [CrossRef]
- Huh, K.M.; Bae, Y.H. Synthesis and characterization of poly(ethylene glycol)/poly(l-lactic acid) alternating multiblock copolymers. Polymer 1999, 40, 6147–6155. [Google Scholar] [CrossRef]
- Luo, W.; Li, S.; Bei, J.; Wang, S. Dependence of morphology on composition of poly(l-lactide)–poly(ethylene glycol) multiblock copolymers. Polym. Adv. Technol. 2002, 13, 233–238. [Google Scholar] [CrossRef]
- Chen, W.; Luo, W.; Wang, S.; Bei, J. Synthesis and properties of poly(l-lactide)–poly(ethylene glycol) multiblock copolymers by coupling triblock copolymers. Polym. Adv. Technol. 2003, 14, 245–253. [Google Scholar] [CrossRef]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Boettcher, C.; Tonnes, K.U. Polylactones: 23. Polymerization of racemic and meso d,l-lactide with various organotin catalysts-stereochemical aspects. Polymer 1992, 33, 2817–2824. [Google Scholar] [CrossRef]
- Thakur, K.A.M.; Kean, R.T.; Hall, E.S.; Kolstad, J.J.; Lindgren, T.A. High-resolution 13C and 1H solution NMR study of poly(lactide). Macromolecules 1997, 30, 2422–2428. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of lactide polymerization with chiral catalysts: New opportunities for stereocontrol using polymer exchange mechanisms. J. Am. Chem. Soc. 2002, 124, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Baick, I.H.; Luciani, C.V.; Park, S.Y.; Lim, T.; Choi, K.Y. Kinetics of reversible oligomerization of l-lactic acid with a SnCl2·2H2O/p-toluenesulfonic acid catalyst. Ind. Eng. Chem. Res. 2012, 51, 16617–16625. [Google Scholar] [CrossRef]
- Miao, P.; Zhao, C.; Xu, G.; Fu, Q.; Tang, W.; Zeng, K.; Wang, Y.; Zhou, H.; Yang, G. Degradation of poly(d,l-lactic acid)-b-poly(ethylene glycol)-b-poly(d,l-lactic acid) copolymer by electron beam radiation. J. Appl. Polym. Sci. 2009, 112, 2981–2987. [Google Scholar]
- Wu, X.; Ghzaoui, A.E.; Li, S. Anisotropic self-assembling micelles prepared by the direct dissolution of PLA/PEG block copolymers with a high PEG fraction. Langmuir 2011, 27, 8000–8008. [Google Scholar] [CrossRef] [PubMed]
- Jie, P.; Venkatraman, S.S.; Min, F.; Freddy, B.Y.; Huat, G.L. Micelle-like nanoparticles of star-branched PEO–PLA copolymers as chemotherapeutic carrier. J. Control. Release 2005, 110, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Z.; Park, S.; Kim, S.H.; Kim, J.H.; Piao, L. Preparation and characterization of PEG/PLA multiblock and triblock copolymer. Bull. Korean Chem. Soc. 2012, 33, 1638–1642. [Google Scholar] [CrossRef]
- Hadjiantoniou, N.A.; Triftaridou, A.I.; Kafouris, D.; Gradzielski, M.; Patrickios, C.S. Synthesis and characterization of amphiphilic multiblock copolymers: Effect of the number of blocks on micellization. Macromolecules 2009, 42, 5492–5498. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chin, I.J.; Jung, J.S. Crystallization behavior of poly(l-lactide)–poly(ethylene glycol) multiblock copolymers. Eur. Polym. J. 1999, 35, 2147–2153. [Google Scholar] [CrossRef]
- Glavas, L.; Olsen, P.; Odelius, K.; Albertsson, A.C. Achieving micelle control through core crystallinity. Biomacromolecules 2013, 14, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Sanabria-Delong, N.; Coburn, J.M.; Tew, G.N.; Bhatia, S.R. Novel drug release profiles from micellar solutions of PLA–PEO–PLA triblock copolymers. J. Control. Release 2006, 112, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.K.; Sanabria-Delong, N.; Tew, G.N.; Bhatia, S.R. Structural characterization of PLA–PEO–PLA solutions and hydrogels: Crystalline vs. amorphous PLA domains. Macromolecules 2008, 41, 1774–1784. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somekawa, S.; Masutani, K.; Hsu, Y.-I.; Mahara, A.; Kimura, Y.; Yamaoka, T. Size-Controlled Nanomicelles of Poly(lactic acid)–Poly(ethylene glycol) Copolymers with a Multiblock Configuration. Polymers 2015, 7, 1177-1191. https://doi.org/10.3390/polym7061177
Somekawa S, Masutani K, Hsu Y-I, Mahara A, Kimura Y, Yamaoka T. Size-Controlled Nanomicelles of Poly(lactic acid)–Poly(ethylene glycol) Copolymers with a Multiblock Configuration. Polymers. 2015; 7(6):1177-1191. https://doi.org/10.3390/polym7061177
Chicago/Turabian StyleSomekawa, Shota, Kazunari Masutani, Yu-I Hsu, Atsushi Mahara, Yoshiharu Kimura, and Tetsuji Yamaoka. 2015. "Size-Controlled Nanomicelles of Poly(lactic acid)–Poly(ethylene glycol) Copolymers with a Multiblock Configuration" Polymers 7, no. 6: 1177-1191. https://doi.org/10.3390/polym7061177