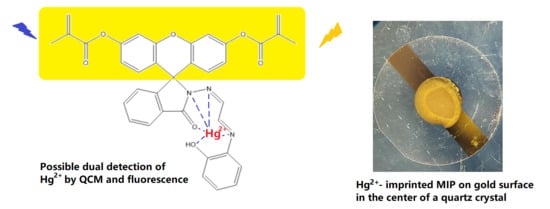
Ion-Imprinted Polymer-Based Sensor for the Detection of Mercury Ions
Abstract
:1. Introduction
1.1. Heavy Metals
1.2. MIPs and IIPs
1.3. QCM
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fluorescein-Based Monomer Synthesis
2.3. Polymerization of Fluorescein-Based Hg-IIP
2.4. Coating of QCM Sensor Chip
2.5. QCM Analysis Procedure
3. Results and Discussion
3.1. Selectivity and Sensitivity of Fluorescein-Based Hg-IIP
3.2. Calibration Plots for Hg-IIP-QCM Method
3.3. LOD, LOQ, and S/N
3.4. Repeatability
3.5. Interference Analysis
3.6. Real Sample Analysis
3.7. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Heavy Metal Poisoning—NORD (National Organization for Rare Disorders). Available online: https://rarediseases.org/rare-diseases/heavy-metal-poisoning/ (accessed on 26 March 2021).
- Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 26 March 2021).
- Liu, Y.; Dong, X.; Sun, J.; Zhong, C.; Li, B.; You, X.; Liu, B.; Liu, Z. Two-photon fluorescent probe for cadmium imaging in cells. Analyst 2012, 137, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, C. Designing of MIP-based QCM sensor for the determination of Cu(II) ions in solution. Sens. Actuators B Chem. 2009, 142, 210–215. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.; Wu, Y.; Meng, M.; Yan, Y.; Li, C. Recent Advances in Ion-Imprinted Membranes: Separation and Detection Via Ion-Selective Recognition. Environ. Sci. Water Res. Technol. 2019, 5, 1626–1653. [Google Scholar] [CrossRef]
- Sellergren, B.; Shea, K. Chiral Ion-Exchange Chromatography: Correlation Between Solute Retention and a Theoretical Ion-Exchange Model Using Ion-Imprinted Polymers. J. Chromatogr. A 1993, 654, 17–28. [Google Scholar] [CrossRef]
- Lavignac, N.; Allender, C.; Brain, K. Current Status of Molecularly Imprinted Polymers as Alternatives to Antibodies in Sorbent Assays. Anal. Chim. Acta 2004, 510, 139145. [Google Scholar] [CrossRef]
- Jégourel, D.; Delépée, R.; Breton, F.; Rolland, A.; Vidal, R.; Agrofoglio, L. Molecularly Imprinted Polymer Of 5-Methyluridine for Solid-Phase Extraction of Pyrimidine Nucleoside Cancer Markers in Urine. Bioorganic Med. Chem. 2008, 16, 8932–8939. [Google Scholar] [CrossRef]
- Tan, Y.; Peng, H.; Liang, C.; Yao, S. A New Assay System for Phenacetin Using Biomimic Bulk Acoustic Wave Sensor with A Molecularly Imprinted Polymer Coating. Sens. Actuators B Chem. 2001, 73, 179–184. [Google Scholar] [CrossRef]
- Shimizu, D.K.; Stephenson, C.J. Molecularly imprinted polymer sensor arrays. Curr. Opin. Chem. Biol. 2010, 14, 743–750. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.; Eren, T.; Atar, N. A Molecular Imprinted Voltammetric Sensor Based on Carbon Nitride Nanotubes: Application to Determination of Melamine. J. Electrochem. Soc. 2016, 163, B588–B593. [Google Scholar] [CrossRef]
- Quartz Crystal Microbalance (QCM)—Nanoscience Instruments. Available online: https://www.nanoscience.com/techniques/quartz-crystal-microbalance/ (accessed on 26 March 2021).
- Kößlinger, C.; Drost, S.; Aberl, F.; Wolf, H.; Koch, S.; Woias, P. A Quartz Crystal Biosensor for Measurement in Liquids. Biosens. Bioelectron. 1992, 7, 397–404. [Google Scholar] [CrossRef] [PubMed]
- BenDov, I. Piezoelectric Immunosensors for Urine Specimens of Chlamydia Trachomatis Employing Quartz Crystal Microbalance Microgravimetric Analyses. Biosens. Bioelectron. 1998, 13, 4–5. [Google Scholar]
- Min, K.; Cho, M.; Han, S.; Shim, Y.; Ku, J.; Ban, C. A Simple and Direct Electrochemical Detection of Interferon-γ Using Its RNA and DNA Aptamers. Biosens. Bioelectron. 2008, 23, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.M.; Zhang, X.B.; Zhang, L.L.; Jin, X.Y.; Huan, S.Y.; Shen, G.L.; Yu, R.Q. An ultrasensitive electrochemical ‘‘turn-on’’ label-free biosensor for Hg2+ with AuNP-functionalized reporter DNA as a signal amplifier. Chem. Commun. 2009, 37, 5633–5635. [Google Scholar] [CrossRef] [PubMed]
- Sui, N.; Liu, F.Y.; Wang, K.; Xie, F.X.; Wang, L.N.; Tang, J.J.; Liu, M.H.; Yu, W.W. Nano Au-Hg amalgam for Hg2+ and H2O2 detection. Sens. Actuators B 2017, 252, 1010–1015. [Google Scholar] [CrossRef]
- The, H.B.; Li, H.Y.; Li, S.F.Y. Highly sensitive and selective detection of Pb2+ ions using a novel and simple DNAzyme-based quartz crystal microbalance with dissipation biosensor. Analyst 2014, 139, 5170–5175. [Google Scholar]
- Zhang, Y.; Chen, L.; Yang, J.; Zhang, Y.; Yuan, M. An “OR-AND” Logic Gate Based Multifunctional Colorimetric Sensor for The Discrimination of Pb2+ And Cd2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118163. [Google Scholar] [CrossRef] [PubMed]
- Water Sanitation and Health. Available online: https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/sanitation-safety/guidelines-for-safe-use-of-wastewater-greywater-and-excreta (accessed on 22 December 2023).







| Sample | Decrease in Frequency (Hz) |
|---|---|
| 1 ppm Hg2+ | 100.1 |
| 1 ppm Hg2+ + 1 ppm Cu2+ | 90.3 |
| 1 ppm Hg2+ + 1 ppm Zn2+ | 85.5 |
| 1 ppm Hg2+ + 1 ppm Pb2+ | 90.9 |
| 1 ppm Hg2+ + 1 ppm Na+ | 85.4 |
| 1 ppm Hg2+ + 1 ppm Ca2+ | 95.4 |
| 1 ppm Hg2+ + 1 ppm Mg2+ | 90.7 |
| Sample | Decrease in Frequency (Hz) |
|---|---|
| 1 ppm Hg2+ | 100.3 |
| 1 ppm Hg2+ + 10 ppm Na+ | 70.4 |
| 1 ppm Hg2+ + 50 ppm Na+ | 60.5 |
| 1 ppm Hg2+ + 100 ppm Na+ | 60.3 |
| 1 ppm Hg2+ + 10 ppm Zn2+ | 80.3 |
| 1 ppm Hg2+ + 50 ppm Zn2+ | 80.6 |
| 1 ppm Hg2+ + 100 ppm Zn2+ | 80.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, K.M.; Lin, X.; Wu, H.; Li, S.F.Y. Ion-Imprinted Polymer-Based Sensor for the Detection of Mercury Ions. Polymers 2024, 16, 652. https://doi.org/10.3390/polym16050652
Low KM, Lin X, Wu H, Li SFY. Ion-Imprinted Polymer-Based Sensor for the Detection of Mercury Ions. Polymers. 2024; 16(5):652. https://doi.org/10.3390/polym16050652
Chicago/Turabian StyleLow, Kit Meng, Xuanhao Lin, Huanan Wu, and Sam Fong Yau Li. 2024. "Ion-Imprinted Polymer-Based Sensor for the Detection of Mercury Ions" Polymers 16, no. 5: 652. https://doi.org/10.3390/polym16050652