Cellulose Nanocrystals Induced Loose and Porous Graphite Phase Carbon Nitride/Porous Carbon Composites for Capturing and Determining of Organochlorine Pesticides from Water and Fruit Juice by Solid-Phase Microextraction
Abstract
:1. Introduction
2. Experimental
2.1. Reagents, Materials, and Instruments
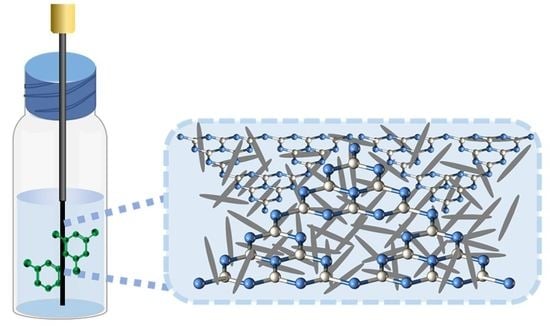
2.2. Synthesis of g-C3N4@PC
2.3. Fabrication of g-C3N4@PC-Coated SPME Fibers
2.4. SPME Procedure and GC-MS Analysis
2.5. Analysis of Real Water and Fruit Juice Samples
3. Results and Discussion
3.1. Characterizations of g-C3N4@PC Materials
3.2. Extraction Performance of g-C3N4@PC-Coated SPME Fibers
3.3. Optimization of g-C3N4@PC-Coated SPME Conditions
3.4. Method Validation and Real Sample Analysis
3.4.1. Validation of Proposed Analytical Method
3.4.2. Analysis of Real Water and Fruit Juice Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braune, B.M.; Outridge, P.M.; Fisk, A.T.; Muir, D.C.; Helm, P.A.; Hobbs, K.; Hoekstra, P.F.; Kuzyk, Z.A.; Kwan, M.; Letcher, R.J.; et al. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: An overview of spatial and temporal trends. Sci. Total Environ. 2005, 351–352, 4–56. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, G.; Xu, J.; Luo, E.; Liu, Y.; Wang, F.; Zhou, H.; Liu, Y.; Zhu, F.; Ouyang, G. In vivo tracing of organochloride and organophosphorus pesticides in different organs of hydroponically grown malabar spinach (Basella alba L.). J. Hazard. Mater. 2016, 316, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Bedmar, F.; Wunderlin, D.A.; Miglioranza, K.S.B. Levels of organochlorine pesticides in soils, mesofauna and streamwater from an agricultural watershed in Argentina. Environ. Earth Sci. 2019, 78, 569. [Google Scholar] [CrossRef]
- Guida, Y.; Carvalho, G.O.; Capella, R.; Pozo, K.; Lino, A.S.; Azeredo, A.; Carvalho, D.F.P.; Braga, A.L.F.; Torres, J.P.M.; Meire, R.O. Atmospheric Occurrence of Organochlorine Pesticides and Inhalation Cancer Risk in Urban Areas at Southeast Brazil. Environ. Pollut. 2021, 271, 116359. [Google Scholar] [CrossRef] [PubMed]
- Attaullah, M.; Yousuf, M.J.; Shaukat, S.; Anjum, S.I.; Ansari, M.J.; Buneri, I.D.; Tahir, M.; Amin, M.; Ahmad, N.; Khan, S.U. Serum organochlorine pesticides residues and risk of cancer: A case-control study. Saudi J. Biol. Sci. 2018, 25, 1284–1290. [Google Scholar] [CrossRef]
- Lin, B.G.; Chen, C.R.; Chen, X.C.; Qiao, J.; Yan, Q.X.; Yang, P.; Chen, W.L.; Li, L.Z.; Qiu, P.C.; Ding, C.; et al. Effects of organochlorine exposure on male reproductive disorders in an electronic waste area of South China. Environ. Int. 2021, 147, 106318. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Sawyna, J.M.; Spivia, W.R.; Radecki, K.; Fraser, D.A.; Lowe, C.G. Association between chronic organochlorine exposure and immunotoxicity in the round stingray (Urobatis halleri). Environ. Pollut. 2017, 223, 42–50. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H.J.; Kim, S.; Choi, G.; Kim, S.; Park, J.; Shim, S.S.; Lee, I.; Kim, S.; Moon, H.B.; et al. Current status of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) exposure among mothers and their babies of Korea-CHECK cohort study. Sci. Total Environ. 2018, 618, 674–681. [Google Scholar] [CrossRef]
- Liu, J.; Qi, S.; Yao, J.; Yang, D.; Xing, X.; Liu, H.; Qu, C. Contamination characteristics of organochlorine pesticides in multimatrix sampling of the Hanjiang River Basin, southeast China. Chemosphere 2016, 163, 35–43. [Google Scholar] [CrossRef]
- Lupi, L.; Bedmar, F.; Wunderlin, D.A.; Miglioranza, K.S.B. Organochlorine pesticides in agricultural soils and associated biota. Environ. Earth Sci. 2016, 75, 519. [Google Scholar] [CrossRef]
- Jin, M.; Fu, J.; Xue, B.; Zhou, S.; Zhang, L.; Li, A. Distribution and enantiomeric profiles of organochlorine pesticides in surface sediments from the Bering Sea, Chukchi Sea and adjacent Arctic areas. Environ. Pollut. 2017, 222, 109–117. [Google Scholar] [CrossRef]
- Islam, M.M.; Avha, N.J.; Ahmed, S.; Akbor, M.A.; Islam, M.S.; Mostafiz, F.; Habibullah-Al-Mamun, M. Trace metals and organochlorine pesticide residues in imported fishes in Bangladesh and human health risk implications. Environ. Sci. Pollut. R. 2022, 29, 17499–17512. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Toms, L.L.; Harden, F.A.; Hobson, P.; White, N.M.; Mengersen, K.L.; Mueller, J.F. Concentrations of organochlorine pesticides in pooled human serum by age and gender. Environ. Res. 2017, 154, 10–18. [Google Scholar] [CrossRef]
- Pastor Belda, M.; Gonzalez-Franco, J.A.; Rubio, R.; Campillo, N.; Hernandez-Cordoba, M.; Torres, C.; Perez-Carceles, M.D.; Vinas, P. Occurrence of Organochlorine Pesticides in Human Tissues Assessed Using a Microextraction Procedure and Gas Chromatography-Mass Spectrometry. J. Anal. Toxicol. 2021, 45, 84–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, L.; Chen, H.; Wang, C.; Xia, Z.; Yuan, C. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TrAC Trend. Anal. Chem. 2016, 80, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Billiard, K.; Dershem, A.; Gionfriddo, E. Implementing Green Analytical Methodologies Using Solid-Phase Microextraction: A Review. Molecules 2020, 25, 5297. [Google Scholar] [CrossRef] [PubMed]
- Onat, B.; Rosales-Solano, H.; Pawliszyn, J. Development of a Biocompatible Solid Phase Microextraction Thin Film Coating for the Sampling and Enrichment of Peptides. Anal. Chem. 2020, 92, 9379–9388. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Long, A.; Feng, S.; Chen, C.-P. In-situ synthesis of carbon dots embedded wrinkled-mesoporous silica microspheres for efficiently capturing and monitoring organochlorine pesticides from water and fruit juice. Sep. Purif. Technol. 2023, 306, 122589. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Chen, C.; Feng, S.; Fan, J. Ultrasound-assisted one-step reduction and self-assembly of carbon dots-reduced graphene oxide: Mechanism investigation and solid phase microextraction of ultra-trace organochlorine pesticides. Chem. Eng. J. 2023, 451, 138569. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Xu, S.; Dong, P.; Long, A.; Xiao, L.; Feng, S.; Chen, C. Cellulose nanocrystal regulated ultra-loose, lightweight, and hierarchical porous reduced graphene oxide hybrid aerogel for capturing and determining organic pollutants from water. Carbon 2023, 204, 94–101. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Souza, I.D.d.; Oliveira, I.G.d.; Grecco, C.F. In vivo solid phase microextraction for bioanalysis. TrAC Trend. Anal. Chem. 2022, 153, 116656. [Google Scholar] [CrossRef]
- Xu, S.; Li, H.; Wu, H.; Xiao, L.; Dong, P.; Feng, S.; Fan, J. A facile cooling-assisted solid-phase microextraction device for solvent-free sampling of polycyclic aromatic hydrocarbons from soil based on matrix solid-phase dispersion technique. Anal. Chim. Acta 2020, 1115, 7–15. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental applications of solid-phase microextraction. TrAC Trend. Anal. Chem. 2019, 112, 1–12. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, J.; Zhang, S.; An, Y.; Hao, L.; Yang, X.; Wang, C.; Wang, Z.; Wu, Q. Novel N-riched covalent organic framework for solid-phase microextraction of organochlorine pesticides in vegetable and fruit samples. Food Chem. 2022, 388, 133007. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Chen, G.; Huang, J.; Zheng, J.; Xu, J.; Liu, S.; Qiu, J.; Yin, L.; Ruan, W.; et al. A graphene oxide-based polymer composite coating for highly-efficient solid phase microextraction of phenols. Anal. Chim. Acta 2018, 1015, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lei, X.; Huang, T.; Su, L.; Zhang, L.; Xie, Z.; Wu, X. Guanidyl-functionalized polyhedral oligomeric silsesquioxane porous hybrid polymer coating for specific solid phase microextraction of phthalate esters in foodstuff. Chem. Eng. J. 2020, 386, 124003. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, J.; Huang, C.; Liu, W.; Tong, P.; Zhang, L. In situ hydrothermal growth of ZnO/g-C3N4 nanoflowers coated solid-phase microextraction fibers coupled with GC-MS for determination of pesticides residues. Anal. Chim. Acta 2016, 934, 122–131. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, X.; Li, H.; Liu, J.; Chang, Q.; Zhang, S.; Wang, C.; Wang, Z. Solid-phase microextraction of organophosphorous pesticides from food samples with a nitrogen-doped porous carbon derived from g-C3N4 templated MOF as the fiber coating. J. Hazard. Mater. 2020, 384, 121430. [Google Scholar] [CrossRef]
- Sun, Y.P.; Chen, J.; Qi, H.Y.; Shi, Y.P. Graphitic carbon nitrides modified hollow fiber solid phase microextraction for extraction and determination of uric acid in urine and serum coupled with gas chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1004, 53–59. [Google Scholar] [CrossRef]
- Ashritha, M.G.; Hareesh, K. A review on Graphitic Carbon Nitride based binary nanocomposites as supercapacitors. J. Energy Storage 2020, 32, 101840. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4 Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wu, T.; Wang, J.; Liang, W.; Zang, X.; Wang, C.; Wu, Q.; Wang, Z. Single layer graphitic carbon nitride-modified graphene composite as a fiber coating for solid-phase microextraction of polycyclic aromatic hydrocarbons. Microchim. Acta 2017, 184, 2171–2180. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, P.; Zhang, X.; Niu, J.; Tian, S.; Lu, M.; Zhu, J.; Cai, Z. Layer-by-layer fabrication of g-C3N4 coating for headspace solid-phase microextraction of food additives followed by gas chromatography-flame ionization detection. Anal. Methods 2018, 10, 322–329. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, P.; Zhang, J.; Li, W.; Zhu, J.; Lu, M.; Cai, Z. Fabrication of nanoscale graphitic carbon nitride/copper oxide hybrid composites coated solid-phase microextraction fibers coupled with gas chromatography for determination of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2018, 1570, 47–55. [Google Scholar] [CrossRef]
- Huang, F.; Lv, J.; Li, H.; Xu, S. Regulation rule of cellulose nanocrystals on thixotropy of hydrogel for water shutoff in horizontal wells. Colloids Surf. A 2022, 643, 128735. [Google Scholar] [CrossRef]
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef]
- Xu, S.; Dong, P.; Liu, H.; Li, H.; Chen, C.; Feng, S.; Fan, J. Lotus-like Ni@NiO nanoparticles embedded porous carbon derived from MOF-74/cellulose nanocrystal hybrids as solid phase microextraction coating for ultrasensitive determination of chlorobenzenes from water. J. Hazard. Mater. 2022, 429, 128384. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.; Long, A.; Li, H.; Chen, C.; Feng, S.; Fan, J. Carbon Dot-Decorated Graphite Carbon Nitride Composites for Enhanced Solid-Phase Microextraction of Chlorobenzenes from Water. Nanomaterials 2022, 12, 335. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient Photocatalytic Overall Water Splitting Induced by the Giant Internal Electric Field of a g-C3N4/rGO/PDIP Z-Scheme Heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, J.; Chai, Y.; Liu, Q.; Ren, J.; Liu, X.; Dai, W.L. CeO2 nanorod/g-C3N4/N-rGO composite: Enhanced visible-light-driven photocatalytic performance and the role of N-rGO as electronic transfer media. Dalton Trans. 2015, 44, 11223–11234. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, Z.; Cui, S.; Guo, X.; Chen, J. Constructing 2D porous graphitic C3N4 nanosheets/nitrogen-doped graphene/layered MoS2 ternary nanojunction with enhanced photoelectrochemical activity. Adv. Mater. 2013, 25, 6291–6297. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Li, J.; Shen, B.; Hong, Z.; Lin, B.; Gao, B.; Chen, Y. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Che, H.; Hu, H.; Hu, W.; Liu, C.; Ai, J.; Dong, H. Decoration of mesoporous Co3O4 nanospheres assembled by monocrystal nanodots on g-C3N4 to construct Z-scheme system for improving photocatalytic performance. Appl. Surf. Sci. 2018, 440, 308–319. [Google Scholar] [CrossRef]
- Gong, X.; Xu, L.; Huang, S.; Kou, X.; Lin, S.; Chen, G.; Ouyang, G. Application of the NU-1000 coated SPME fiber on analysis of trace organochlorine pesticides in water. Anal. Chim. Acta 2022, 1218, 339982. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Yang, H.; Zheng, J.; Qiu, J.; Xu, J.; Tong, Y.; Zhu, F.; Ouyang, G. Hierarchical Graphene coating for highly sensitive solid phase microextraction of organochlorine pesticides. Talanta 2016, 160, 217–224. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Zheng, J.; Jiang, R.; Zhu, F.; Luan, T.; Ouyang, G. Mesoporous TiO2 nanoparticles for highly sensitive solid-phase microextraction of organochlorine pesticides. Anal. Chim. Acta 2015, 878, 109–117. [Google Scholar] [CrossRef]
- Ke, Y.; Zhu, F.; Zeng, F.; Luan, T.; Su, C.; Ouyang, G. Preparation of graphene-coated solid-phase microextraction fiber and its application on organochlorine pesticides determination. J. Chromatogr. A 2013, 1300, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Liu, P.; Zhou, W.; Jia, Q. Preparation of porous aromatic framework/ionic liquid hybrid composite coated solid-phase microextraction fibers and their application in the determination of organochlorine pesticides combined with GC-ECD detection. Analyst 2016, 141, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Merib, J.; Simão, V.; Dias, A.N.; Carasek, E. Simultaneous determination of trihalomethanes and organochlorine pesticides in water samples by direct immersion-headspace-solid phase microextraction. J. Chromatogr. A 2013, 1321, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Castell, E.; Belenguer-Sapiña, C.; Amorós, P.; El Haskouri, J.; Herrero-Martínez, J.M.; Mauri-Aucejo, A.R. Mesoporous silica sorbent with gold nanoparticles for solid-phase extraction of organochlorine pesticides in water samples. J. Chromatogr. A 2022, 1662, 462729. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; Rocío-Bautista, P.; Pino, V.; Jiménez-Moreno, F.; Jiménez-Abizanda, A.I. Gold nanoparticles based solid-phase microextraction coatings for determining organochlorine pesticides in aqueous environmental samples. J. Sep. Sci. 2017, 40, 2009–2021. [Google Scholar] [CrossRef]
- Huang, Z.; Chua, P.E.; Lee, H.K. Carbonized polydopamine as coating for solid-phase microextraction of organochlorine pesticides. J. Chromatogr. A 2015, 1399, 8–17. [Google Scholar] [CrossRef]
- Li, S.; Lu, C.; Zhu, F.; Jiang, R.; Ouyang, G. Preparation of C18 composite solid-phase microextraction fiber and its application to the determination of organochlorine pesticides in water samples. Anal. Chim. Acta 2015, 873, 57–62. [Google Scholar] [CrossRef]






| Analytes | Linear Ranges (ng L−1) | R2 | LOD (ng L−1) | LOQ (ng L−1) | RSD (%) | |
|---|---|---|---|---|---|---|
| One Fiber (n = 7) | Fiber-to-Fiber (n = 5) | |||||
| ETR | 0.1–1600 | 0.9995 | 0.0224 | 0.0749 | 5.2 | 8.9 |
| CHN | 0.1–1600 | 0.9960 | 0.0942 | 0.3140 | 7.3 | 9.2 |
| TRI | 0.1–1600 | 0.9913 | 0.0706 | 0.2355 | 6.4 | 8.5 |
| HCB | 0.1–1600 | 0.9972 | 0.0565 | 0.1884 | 8.9 | 10.7 |
| CHT | 0.1–1600 | 0.9978 | 0.0668 | 0.2228 | 7.6 | 9.4 |
| CHP | 0.1–1600 | 0.9960 | 0.0141 | 0.0471 | 9.3 | 9.7 |
| α-CHD | 0.1–1600 | 0.9933 | 0.0471 | 0.1570 | 6.5 | 10.1 |
| γ-CHD | 0.1–1600 | 0.9917 | 0.0565 | 0.1884 | 5.7 | 10.6 |
| Analytes | Linear Ranges (ng L−1) | R2 | LOD (ng L−1) | LOQ (ng L−1) | RSD (%) | |
|---|---|---|---|---|---|---|
| One Fiber (n = 7) | Fiber-to-Fiber (n = 5) | |||||
| ETR | 0.1–1000 | 0.9924 | 0.0777 | 0.2591 | 4.9 | 7.9 |
| CHN | 0.1–500 | 0.9930 | 0.0282 | 0.0942 | 7.6 | 8.2 |
| TRI | 0.1–500 | 0.9904 | 0.0403 | 0.1345 | 5.4 | 9.5 |
| HCB | 0.1–500 | 0.9929 | 0.0256 | 0.0856 | 7.8 | 8.7 |
| CHT | 0.1–1000 | 0.9982 | 0.0565 | 0.1884 | 8.4 | 9.6 |
| CHP | 0.1–1000 | 0.9994 | 0.0314 | 0.1046 | 9.6 | 8.7 |
| α-CHD | 0.1–1000 | 0.9993 | 0.0245 | 0.0819 | 10.5 | 10.3 |
| γ-CHD | 0.1–1000 | 0.9967 | 0.0282 | 0.0941 | 6.7 | 10.1 |
| Analytical Methods | Coating Materials | Linear Ranges (ng L–1) | LOD (ng L–1) | LOQ (ng L–1) | Reference |
|---|---|---|---|---|---|
| SPME/GC-MS | mesoporous TiO2 | 5–5000 | 0.08–0.60 | 0.27–2.00 | [50] |
| HS-SPME/GC-ECD | porous aromatic framework/ionic liquid | 1000–500,000 | 110–290 | 350–930 | [51] |
| SPME/GC-MS | sol–gel–graphene | 10–100,000 | 0.19–18.30 | – | [52] |
| HS-SPME/GC-MS | DVB/CAR/PDMS | 20–100,000 | 20–230 | 20–770 | [53] |
| SPE/GC-ECD | Au/Ti-UVM-7 | 400–100,000 | 0.30–20 | 1–61 | [54] |
| SPME/GC-ECD | AuNPs | 560–10,000 | 130–240 | 440–810 | [55] |
| SPME/GC-MS | carbonized polydopamine | 10–50,000 | 1.40–15 | – | [56] |
| SPME/GC-MS | C18 composite | 2–500 | 0.059–0.151 | – | [57] |
| SPME/GC-MS | g-C3N4@PC | 0.1–1600 | 0.0141–0.0942 | 0.0471–0.3140 | This work |
| Analytes | Tap Water (1#) | Weihe River (2#) | Rain Water (3#) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 2 ng L–1) | Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 2 ng L–1) | Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 0.2 ng L–1) | |
| ETR | ND | – | 125.5 | ND | – | 94.1 | ND | – | 93.5 |
| CHN | ND | – | 104.8 | ND | – | 93.4 | ND | – | 107.3 |
| TRI | ND | – | 96.4 | ND | – | 90.6 | 10.9 | – | 94.1 |
| HCB | ND | – | 122.4 | ND | – | 93.9 | ND | – | 95.7 |
| CHT | ND | – | 103.3 | ND | – | 101.2 | ND | – | 98.8 |
| CHP | ND | – | 93.2 | ND | – | 95.4 | ND | – | 93.3 |
| α-CHD | ND | – | 98.1 | ND | – | 98.3 | ND | – | 102.7 |
| γ-CHD | ND | – | 95.6 | ND | – | 97.6 | ND | – | 91.4 |
| Analytes | Grape Juice(4#) | Orange Juice(5#) | Peach Juice(6#) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 1 ng L–1) | Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 1 ng L–1) | Found (ng L–1) | RSD (%, n = 3) | Recoveries (%, Spiked with 0.1 ng L–1) | |
| ETR | ND | – | 92.3 | ND | – | 92.7 | ND | – | 96.8 |
| CHN | ND | – | 94.4 | ND | – | 95.2 | ND | – | 97.2 |
| TRI | ND | – | 97.3 | ND | – | 98.2 | 10.9 | – | 95.6 |
| HCB | ND | – | 94.8 | ND | – | 95.6 | ND | – | 106.3 |
| CHT | ND | – | 107.9 | ND | – | 113.8 | ND | – | 98.2 |
| CHP | ND | – | 119.5 | ND | – | 94.3 | ND | – | 93.9 |
| α-CHD | ND | – | 95.8 | ND | – | 92.6 | ND | – | 96.1 |
| γ-CHD | ND | – | 98.7 | ND | – | 108.3 | ND | – | 95.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Dong, P.; Long, A.; Feng, S.; Fan, J.; Xu, S. Cellulose Nanocrystals Induced Loose and Porous Graphite Phase Carbon Nitride/Porous Carbon Composites for Capturing and Determining of Organochlorine Pesticides from Water and Fruit Juice by Solid-Phase Microextraction. Polymers 2023, 15, 2218. https://doi.org/10.3390/polym15092218
Li H, Dong P, Long A, Feng S, Fan J, Xu S. Cellulose Nanocrystals Induced Loose and Porous Graphite Phase Carbon Nitride/Porous Carbon Composites for Capturing and Determining of Organochlorine Pesticides from Water and Fruit Juice by Solid-Phase Microextraction. Polymers. 2023; 15(9):2218. https://doi.org/10.3390/polym15092218
Chicago/Turabian StyleLi, Huimin, Panlong Dong, Anying Long, Suling Feng, Jing Fan, and Shengrui Xu. 2023. "Cellulose Nanocrystals Induced Loose and Porous Graphite Phase Carbon Nitride/Porous Carbon Composites for Capturing and Determining of Organochlorine Pesticides from Water and Fruit Juice by Solid-Phase Microextraction" Polymers 15, no. 9: 2218. https://doi.org/10.3390/polym15092218