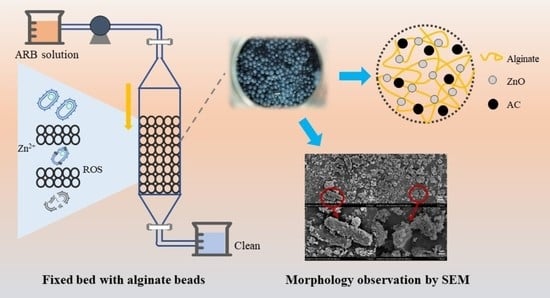
3D ZnO/Activated Carbon Alginate Beads for the Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO/AC Alginate Beads
2.3. Characterization of ZnO/AC Alginate Beads
2.4. The Removal Efficiency of ARB by ZnO/AC Alginate Beads
2.5. Application of ZnO/AC Alginate Beads in Fixed Bed
2.6. Absolute Abundance of Antibiotic Resistance Genes
2.7. Mechanism of ARB Removal
2.7.1. Microbial Morphology Using SEM
2.7.2. Live/Dead Fluorescence Assay
2.7.3. Lipid Peroxidation Analysis
2.7.4. Oxidative Stress Measurements
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnO/AC Alginate Beads
3.2. The Removal Efficiency of ARB Using ZnO/AC Alginate Beads
3.3. Application of ZnO/AC Alginate Beads in Fixed Bed
3.3.1. Removal Efficiency of ARB under Different Parameters
3.3.2. Removal Efficiency of ARGs under Different Parameters
3.3.3. The Performance Analysis of Fixed Bed
3.4. Mechanism of ARB Removal
3.4.1. Morphology Observation Using SEM
3.4.2. Investigation of Cell Damage by CLSM
3.4.3. Lipid Peroxidation and Oxidative Stress Response
3.4.4. Contribution of Inactivation and Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Do, T.A.R.; Andersen, B.M.; Banach, D.B.; Bryant, K.A. Reliability of nonlocalizing signs and symptoms as indicators of the presence of infection in nursing-home residents. Infect. Control Hosp. Epidemiol. 2022, 43, 417–426. [Google Scholar] [CrossRef]
- El-hack, M.E.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird ’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, Z.; Zhu, L.; Han, Y.; Lin, Z.; Feng, W.; Liu, Y.; Shuai, X.; Chen, H. Antibiotic resistance genes and microcystins in a drinking water treatment plant. Environ. Pollut. 2020, 258, 113718. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, Z.; Zhu, L.; Meng, L.; Shuai, X.; Sun, Y.; Chen, H. Behavior of antibiotic resistance genes in a wastewater treatment plant with different upgrading processes. Sci. Total Environ. 2021, 771, 144814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Lin, Z.; Shuai, X.; Zhu, L.; Xu, L.; Meng, L.; Sun, Y.; Chen, H. Spread of antibiotic resistance genes and microbiota in airborne particulate matter, dust, and human airways in the urban hospital. Environ. Int. 2021, 153, 106501. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in Northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The World Health Report: A Safer Future-Global Public Health Security in the 21st Century; World Health Organization: Geneva, Switzerland, 2007; pp. 22–23. [Google Scholar]
- Affek, K.; Muszyński, A.; Doskocz, N.; Załęska-Radziwiłł, M. Ecotoxicological effects of disinfection of treated wastewater. Desalin. Water Treat. 2021, 233, 190–198. [Google Scholar] [CrossRef]
- Liang, C.; Wei, D.; Yan, W.; Zhang, S.; Shi, J.; Liu, L. Fates of intracellular and extracellular antibiotic resistance genes during the cattle farm wastewater treatment process. Bioresour. Technol. 2022, 344, 126272. [Google Scholar] [CrossRef]
- Hafeez, A.; Shamair, Z.; Shezad, N.; Javed, F.; Fazal, T.; Rehman, S.U.; Bazmi, A.A.; Rehman, F. Solar powered decentralized water systems: A cleaner solution of the industrial wastewater treatment and clean drinking water supply challenges. J. Clean. Prod. 2021, 289, 125717. [Google Scholar] [CrossRef]
- Zheng, J.; Su, C.; Zhou, J.; Xu, L.; Qian, Y.; Chen, H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017, 317, 309–316. [Google Scholar] [CrossRef]
- Stange, C.; Sidhu, J.P.S.; Toze, S.; Tiehm, A. Comparative removal of antibiotic resistance genes during chlorination, ozonation, and UV treatment. Int. J. Hyg. Environ. Health 2019, 222, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mitch, W.A. Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shuai, X.; Xu, L.; Sun, Y.; Lin, Z.; Zhou, Z.; Meng, L.; Chen, H. Mechanisms underlying the effect of chlorination and UV disinfection on VBNC state Escherichia coli isolated from hospital wastewater. J. Hazard. Mater. 2022, 423, 127228. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-emre, E.S.; Bahng, J.H.; Kotov, N.A.; Vanepps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin. Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Eskikaya, O.; Ozdemir, S.; Tollu, G.; Dizge, N.; Ramaraj, R. Synthesis of two different zinc oxide nanoflowers and comparison of antioxidant and photocatalytic activity. Chemosphere 2022, 306, 135389. [Google Scholar] [CrossRef]
- Zewde, D.; Geremew, B. Biosynthesis of ZnO nanoparticles using Hagenia abyssinica leaf extracts; their photocatalytic and antibacterial activities. Environ. Pollut. Bioavailab. 2022, 34, 224–235. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Wan, Z.; Cho, D.W.; Tsang, D.C.W.; Li, M.; Sun, T.; Verpoort, F. Concurrent adsorption and micro-electrolysis of Cr(VI) by nanoscale zerovalent iron/biochar/Ca-alginate composite. Environ. Pollut. 2019, 247, 410–420. [Google Scholar] [CrossRef]
- Li, Y.; Wen, J.; Xue, Z.; Yin, X.; Yuan, L.; Yang, C. Removal of Cr(VI) by polyaniline embedded polyvinyl alcohol/sodium alginate beads – Extension from water treatment to soil remediation. J. Hazard. Mater. 2021, 426, 127809. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Hee, S.; Toborek, M. Treatment of antibiotic-resistant bacteria by encapsulation of ZnO nanoparticles in an alginate biopolymer: Insights into treatment mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.; Sun, X.; Liu, J.; Song, C.; Wang, S.; Javed, A. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ. 2018, 639, 560–569. [Google Scholar] [CrossRef]
- Constance, S.; Sinha, S.; Maity, A. Synthesis and characterization of alginate beads encapsulated zinc oxide nanoparticles for bacteria disinfection in water. J. Colloid Interface Sci. 2018, 512, 686–692. [Google Scholar] [CrossRef]
- Zhao, W.B.; Du, M.R.; Liu, K.K.; Zhou, R.; Ma, R.N.; Jiao, Z.; Zhao, Q.; Shan, C.X. Hydrophilic ZnO Nanoparticles@Calcium Alginate Composite for Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 13305–13315. [Google Scholar] [CrossRef]
- Pooi, C.K. Review of low-cost point-of-use water treatment systems for developing communities. NPJ Clean Water 2018, 1, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, J.; Li, X.; Han, Y.; Meng, H.; Wang, T.; Zhang, X. Simple synthesis of ZnO nanoflowers and its photocatalytic performances toward the photodegradation of metamitron. Mater. Res. Bull. 2016, 76, 235–239. [Google Scholar] [CrossRef]
- Lebel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2′,7′-Dichlorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Li, S.; Tong, Y.; Ye, B. Synthesis of magnetic microspheres with sodium alginate and activated carbon for removal of methylene blue. Materials 2017, 10, 84. [Google Scholar] [CrossRef]
- Shim, J.; Kumar, M.; Goswami, R.; Mazumder, P.; Oh, B.T.; Shea, P.J. Removal of p-cresol and tylosin from water using a novel composite of alginate, recycled MnO2 and activated carbon. J. Hazard. Mater. 2019, 364, 419–428. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Volume 1. [Google Scholar]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Motshekga, S.C.; Sinha, S. Highly efficient inactivation of bacteria found in drinking water using chitosan-bentonite composites: Modelling and breakthrough curve analysis. Water Res. 2017, 111, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, G.; An, C.; Huang, J.; Xin, X.; Chen, X.; Hong, Y.; Song, P. Removal of Escherichia coli from water using functionalized porous ceramic disk filter coated with Fe/TiO2 nano-composites. J. Water Process. Eng. 2020, 33, 101013. [Google Scholar] [CrossRef]
- Huang, J.; Huang, G.; An, C.; He, Y.; Yao, Y.; Zhang, P. Performance of ceramic disk filter coated with nano ZnO for removing Escherichia coli from water in small rural and remote communities of developing regions. Environ. Pollut. 2018, 238, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Singleton, C.M.; Petriglieri, F.; Kristensen, J.M.; Kirkegaard, R.H.; Michaelsen, T.Y.; Andersen, M.H.; Kondrotaite, Z.; Karst, S.M.; Dueholm, M.S.; Nielsen, P.H.; et al. Connecting structure to function with the recovery of over 1000 high-quality metagenome-assembled genomes from activated sludge using long-read sequencing. Nat. Commun. 2021, 12, 2009. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater- Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]
- Banerjee, S.; Joshi, S.R.; Mandal, T.; Halder, G. Application of zirconium caged activated biochar alginate beads towards deionization of Cr (VI) laden water in a fixed bed column reactor. J. Environ. Chem. Eng. 2018, 6, 4018–4029. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, L.; Zhu, J.; Wu, H.; Li, W.; Sun, Q. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard. Mater. 2021, 402, 123542. [Google Scholar] [CrossRef]
- Basu, A.; Behera, M.; Maharana, R.; Kumar, M.; Tripathy, S.K. To unsnarl the mechanism of disinfection of Escherichia coli via visible light assisted heterogeneous photo-Fenton reaction in presence of biochar supported maghemite nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104620. [Google Scholar] [CrossRef]
- Misra, A.J.; Basu, A.; Behera, S.K.; Mishra, A.; Lundborg, C.S.; Tripathy, S.K. Point-of-use photocatalytic device for water disinfection under visible light using ZnO/Gypsum@alginate beads. J. Environ. Chem. Eng. 2022, 10, 107520. [Google Scholar] [CrossRef]
- Hye, S.; Hoon, S.; Ho, J.; Ho, J.; Jeong, E.; Ji, Y.; Ha, E. Inactivation of Escherichia coli and Staphylococcus aureus on contaminated perilla leaves by Dielectric Barrier Discharge (DBD) plasma treatment. Arch. Biochem. Biophys. 2018, 643, 32–41. [Google Scholar] [CrossRef]
- Saeidnia, S.; Abdollahi, M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharmacol. 2013, 273, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yu, X.; Zhou, Z.; Zhou, J.; Shuai, X.; Lin, Z.; Chen, H. 3D ZnO/Activated Carbon Alginate Beads for the Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes. Polymers 2023, 15, 2215. https://doi.org/10.3390/polym15092215
Liu Z, Yu X, Zhou Z, Zhou J, Shuai X, Lin Z, Chen H. 3D ZnO/Activated Carbon Alginate Beads for the Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes. Polymers. 2023; 15(9):2215. https://doi.org/10.3390/polym15092215
Chicago/Turabian StyleLiu, Zhe, Xi Yu, Zhenchao Zhou, Jinyu Zhou, Xinyi Shuai, Zejun Lin, and Hong Chen. 2023. "3D ZnO/Activated Carbon Alginate Beads for the Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes" Polymers 15, no. 9: 2215. https://doi.org/10.3390/polym15092215