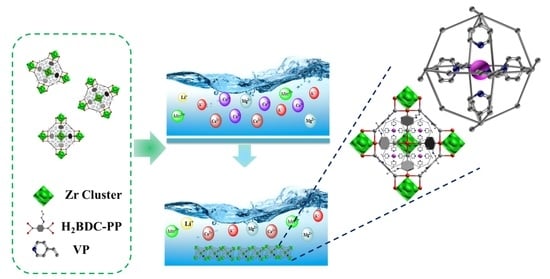
Synthesis and Application of a Novel Metal–Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MIIP
2.3. Characterisation
2.4. Bath Sorption Experiments
2.5. DFT Calculations
3. Results and Discussion
3.1. Characterisations
3.2. Sorption Experiments
3.2.1. Effect of pH
3.2.2. Sorption Kinetics
3.2.3. Sorption Thermodynamics
3.2.4. Sorption Isotherms
3.2.5. Selectivity and Reusability
3.3. Sorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, F.; Yin, Y.; Wang, J. Removal of cobalt ions from simulated radioactive wastewater by vacuum membrane distillation. Prog. Nucl. Energy 2018, 103, 20–27. [Google Scholar] [CrossRef]
- Kazemi, Z.; Marahel, F.; Hamoule, T.; Mombeni Goodajdar, B. Removal of Ni(II) and Co(II) ions from aqueous solutions utilizing Origanum majorana-capped silver nanoparticles. Desalin. Water Treat. 2021, 213, 381–394. [Google Scholar] [CrossRef]
- Gras, M.; Duclos, L.; Schaeffer, N.; Mogilireddy, V.; Svecova, L.; Chaînet, E.; Billard, I.; Papaiconomou, N. A Comparison of Cobalt and Platinum Extraction in Hydrophobic and Hydrophilic Ionic Liquids: Implication for Proton Exchange Membrane Fuel Cell Recycling. ACS Sustain. Chem. Eng. 2020, 8, 15865–15874. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Mubarok, M.Z.; Magnalita, A.; Vaughan, J.; Sugiarto, A.T. Processing mixed nickel-cobalt hydroxide precipitate by sulfuric acid leaching followed by selective oxidative precipitation of cobalt and manganese. Hydrometallurgy 2020, 191, 105185. [Google Scholar] [CrossRef]
- Xing, M.; Xu, L.J.; Wang, J.L. Mechanism of Co(II) adsorption by zero valent iron/graphene nanocomposite. J. Hazard. Mater. 2016, 301, 286–296. [Google Scholar] [CrossRef]
- Awual, M.R.; Alharthi, N.H.; Hasan, M.M.; Karim, M.R.; Islam, A.; Znad, H.; Hossain, M.A.; Halim, M.E.; Rahman, M.M.; Khaleque, M.A. Inorganic-organic based novel nano-conjugate material for effective cobalt(II) ions capturing from wastewater. Chem. Eng. J. 2017, 324, 130–139. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, P.; Yan, S.; Liu, Y.; Zhang, G. Effective removal of radioactive cobalt from aqueous solution by a layered metal sulfide adsorbent: Mechanism, adsorption performance, and practical application. Sep. Purif. Technol. 2021, 256, 117775. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R.; Christa, J. Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt(II) from nuclear industry wastewater samples. J. Colloid Interface Sci. 2016, 467, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Jiang, S.; Ma, X.; Wei, B. Removal and recovery of cobalt from Co(II)–containing water samples by dithiocarboxyl polyethyleneimine. Sep. Purif. Technol. 2020, 251, 117338. [Google Scholar] [CrossRef]
- Yuan, G.; Yu, Y.; Li, J.; Jiang, D.; Gu, J.; Tang, Y.; Qiu, H.; Xiong, W.; Liu, N. Facile fabrication of a noval melamine derivative-doped UiO-66 composite for enhanced Co(II) removal from aqueous solution. J. Mol. Liq. 2021, 328, 115484. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Q.; Li, S.; Fei, J.; Zhou, J.; Shan, S.; Li, Z.; Li, H.; Chen, S. Highly efficient removal of quinolones by using the easily reusable MOF derived-carbon. J. Hazard. Mater. 2022, 423, 127181. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Liang, K.; Lin, J.; Li, J.; Li, L.; Kang, L.; Yu, Y.; Xi, H. Application of hierarchically porous metal-organic frameworks in heterogeneous catalysis: A review. Sci. China Mater. 2022, 65, 298–320. [Google Scholar] [CrossRef]
- Mirzaei, K.; Jafarpour, E.; Shojaei, A.; Khasraghi, S.S.; Jafarpour, P. An investigation on the influence of highly acidic media on the microstructural stability and dye adsorption performance of UiO-66. Appl. Surf. Sci. 2023, 618, 156531. [Google Scholar] [CrossRef]
- Ahmadipouya, S.; Mousavi, S.A.; Shokrgozar, A.; Mousavi, D.V. Improving dye removal and antifouling performance of polysulfone nanofiltration membranes by incorporation of UiO-66 metal-organic framework. J. Environ. Chem. Eng. 2022, 10, 107535. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Yue, C.; Song, L.; Lv, Y.; Liu, F.; Li, A. Insight into the efficient co-removal of Cr(VI) and Cr(III) by positively charged UiO-66-NH2 decorated ultrafiltration membrane. Chem. Eng. J. 2021, 404, 126546. [Google Scholar] [CrossRef]
- Su, S.; Che, R.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Jing, X.; Wang, J. Zeolitic Imidazolate Framework-67: A promising candidate for recovery of uranium (VI) from seawater. Colloids Surf. A 2018, 547, 73–80. [Google Scholar] [CrossRef]
- Abdollahi, N.; Akbar Razavi, S.A.; Morsali, A.; Hu, M.L. High capacity Hg(II) and Pb(II) removal using MOF-based nanocomposite: Cooperative effects of pore functionalization and surface-charge modulation. J. Hazard. Mater. 2020, 387, 121667. [Google Scholar] [CrossRef]
- Hu, H.; Ren, Z.; Xi, Y.; Fang, L.; Fang, D.; Yang, L.; Shao, P.; Shi, H.; Yu, K.; Luo, X. Insights into the role of cross-linking agents on polymer template effect: A case study of anionic imprinted polymers. Chem. Eng. J. 2021, 420, 129611. [Google Scholar] [CrossRef]
- Biswas, T.K.; Yusoff, M.M.; Sarjadi, M.S.; Arshad, S.E.; Musta, B.; Rahman, M.L. Ion-imprinted polymer for selective separation of cobalt, cadmium and lead ions from aqueous media. Sep. Sci. Technol. 2019, 56, 671–680. [Google Scholar] [CrossRef]
- Adibmehr, Z.; Faghihian, H. Preparation of highly selective magnetic cobalt ion-imprinted polymer based on functionalized SBA-15 for removal Co(2+) from aqueous solutions. J. Environ. Health Sci. Eng. 2019, 17, 1213–1225. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Liu, J.; Tu, H.; Liao, J.; Yang, J.; Yang, Y.; Wang, D.; Liu, N. Schiff base anchored on metal-organic framework for Co (II) removal from aqueous solution. Chem. Eng. J. 2017, 326, 691–699. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH2 by 2,5-Dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lan, T.; Li, M.; Yuan, G.; Li, F.; Liao, J.; Yang, J.; Yang, Y.; Liu, N. Removal of Co(II) from Aqueous Solutions by Pyridine Schiff Base-Functionalized Zirconium-Based MOFs: A Combined Experimental and DFT Study on the Effect of ortho-, meta-, and para-Substitution. J. Chem. Eng. Data 2020, 66, 749–760. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Zhang, J.; Sun, J. One-pot synthesis of pyridine-based ionic hyper-cross-linked polymers with hierarchical pores for efficient CO2 capture and catalytic conversion. Chem. Eng. J. 2022, 427, 131633. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]
- Yuan, G.; Tu, H.; Li, M.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. Glycine derivative-functionalized metal-organic framework (MOF) materials for Co(II) removal from aqueous solution. Appl. Surf. Sci. 2019, 466, 903–910. [Google Scholar] [CrossRef]
- Khan, A.A.; Sing, R.P. Adsorption Thermodynamics of Carbofuran on Sn (IV) Arsenosilicate in H+, Na+ and Ca2+ Forms. Colloids Surf. 1987, 24, 33–42. [Google Scholar] [CrossRef]
- Khoddami, N.; Shemirani, F. A new magnetic ion-imprinted polymer as a highly selective sorbent for determination of cobalt in biological and environmental samples. Talanta 2016, 146, 244–252. [Google Scholar] [CrossRef]
- Lee, H.-K.; Choi, J.-W.; Choi, S.-J. Magnetic ion-imprinted polymer based on mesoporous silica for selective removal of Co(II) from radioactive wastewater. Sep. Sci. Technol. 2020, 56, 1842–1852. [Google Scholar] [CrossRef]
- Nishad, P.A.; Bhaskarapillai, A.; Velmurugan, S.; Narasimhan, S.V. Cobalt (II) imprinted chitosan for selective removal of cobalt during nuclear reactor decontamination. Carbohydr. Polym. 2012, 87, 2690–2696. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Li, M.; Zeng, Y.; Tu, H.; Liao, J.; Yang, J.; Yang, Y.; Liu, N. Removal of Co(II) from aqueous solution with functionalized metal–organic frameworks (MOFs) composite. J. Radioanal. Nucl. Chem. 2019, 322, 827–838. [Google Scholar] [CrossRef]
- Lai, W.; Zhang, K.; Shao, P.; Yang, L.; Ding, L.; Pavlostathis, S.G.; Shi, H.; Zou, L.; Liang, D.; Luo, X. Optimization of adsorption configuration by DFT calculation for design of adsorbent: A case study of palladium ion-imprinted polymers. J. Hazard. Mater. 2019, 379, 120791. [Google Scholar] [CrossRef]
- Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 1987, 86, 866–872. [Google Scholar] [CrossRef]
- Xia, M.; Yang, X.; Chai, Z.; Wang, D. Stronger hydration of Eu(III) impedes its competition against Am(III) in binding with N-donor extractants. Inorg. Chem. 2020, 59, 6267–6278. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, R.; Liu, Y.; Meng, M.J.; Meng, X.G.; Hu, Z.Y.; Song, Z.L. Preparation of ion-imprinted mesoporous silica SBA-15 functionalized with triglycine for selective adsorption of Co(II). Colloid Surf. A 2013, 436, 693–703. [Google Scholar] [CrossRef]







| Samples | Surface Area (m2∙g−1) | Pore Diameter (nm) | Pore Volume (cc∙g−1) |
|---|---|---|---|
| UiO-66-NH2 | 964.2 | 3.8 | 0.35 |
| UiO-66-PP | 812.6 | 3.7 | 0.32 |
| MNIP | 399.1 | 4.0 | 0.20 |
| MIIP | 672.8 | 3.7 | 0.28 |
| Samples | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 | R2 | kint | C | R2 | |
| MNIP | 46.7 | 6.18 × 10−2 | 0.977 | 53.2 | 1.31 × 10−4 | 0.984 | 1.25 | 10.7 | 0.753 |
| MIIP | 62.5 | 5.98 × 10−2 | 0.981 | 71.9 | 9.48 × 10−5 | 0.990 | 1.69 | 13.8 | 0.795 |
| Samples | T (K) | ln Kd | ΔG0 (kJ∙mol−1) | ΔH0 (kJ∙mol−1) | ΔS0 (J∙mol−1∙K−1) |
|---|---|---|---|---|---|
| MNIP | 288 | 2.38 | −6.0 | 40.2 | 160.4 |
| 298 | 3.36 | −7.6 | |||
| 308 | 3.46 | −9.2 | |||
| MIIP | 288 | 3.77 | −8.9 | 20.5 | 102.1 |
| 298 | 3.89 | −9.9 | |||
| 308 | 4.33 | −10.9 |
| Samples | T | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| KL | qmax | R2 | 1/n | KF | R2 | ||
| MNIP | 288 K | 0.064 | 111.1 | 0.998 | 0.456 | 14.9 | 0.983 |
| 298 K | 0.114 | 120.2 | 0.999 | 0.360 | 26.6 | 0.972 | |
| 308 K | 0.104 | 139.9 | 0.995 | 0.365 | 29.8 | 0.989 | |
| MIIP | 288 K | 0.126 | 130.4 | 0.998 | 0.332 | 32.4 | 0.987 |
| 298 K | 0.131 | 167.5 | 0.994 | 0.351 | 39.8 | 0.975 | |
| 308 K | 0.150 | 181.5 | 0.994 | 0.324 | 48.5 | 0.989 | |
| Sorbents | T (K) | pH | qe (mg∙g−1) | Ref. |
|---|---|---|---|---|
| Fe3O4@TiO2@SiO2-IIP | / | 8.0 | 35.2 | [31] |
| Co(II)-MIIP | 298 | 8.0 | 74.0 | [20] |
| Mag@silica-CIP | 298 | 5.0 | 78.9 | [32] |
| MIP | / | 4.8 | 92.2 | [33] |
| IIPs | / | 6.0 | 105.0 | [19] |
| MIIP | 308 | 8.3 | 181.5 | This work |
| Metal Ions | MIIP | MNIP | ||
|---|---|---|---|---|
| Kd | k | Kd | k | |
| Co(II) | 60.42 | 3.48 | ||
| Li(I) | 1.36 | 44.31 | 1.11 | 3.12 |
| K(I) | 1.82 | 33.19 | 1.55 | 2.24 |
| Mg(II) | 5.58 | 10.84 | 3.66 | 0.95 |
| Ca(II) | 2.18 | 27.71 | 1.48 | 2.34 |
| Mn(II) | 6.39 | 9.45 | 2.66 | 1.31 |
| Ba(II) | 3.72 | 16.25 | 3.35 | 1.04 |
| Cd(II) | 7.95 | 7.60 | 3.21 | 1.08 |
| Path | Sorption Reaction | ΔG | ΔE |
|---|---|---|---|
| 1 | Co(H2O)62+ + VP = Co(VP)(H2O)52+ + H2O | −4.85 | −10.95 |
| 2 | Co(H2O)62+ + 2 VP = Co(VP)2(H2O)42+ + 2 H2O | −8.46 | −19.11 |
| 3 | Co(H2O)62+ + 3 VP = Co(VP)3(H2O)32+ + 3 H2O | −14.02 | −31.96 |
| 4 | Co(H2O)62+ + 4 VP = Co(VP)4(H2O)22+ + 4 H2O | −10.05 | −35.01 |
| 5 | Co(H2O)62+ + 5 VP = Co(VP)5H2O2+ + 5 H2O | −23.26 | −51.74 |
| 6 | Co(H2O)62+ + 6 VP = Co(VP)62+ + 6 H2O | −23.31 | −59.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Lan, T.; Yuan, G.; Duan, C.; Pu, X.; Liu, N. Synthesis and Application of a Novel Metal–Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater. Polymers 2023, 15, 2150. https://doi.org/10.3390/polym15092150
Yu L, Lan T, Yuan G, Duan C, Pu X, Liu N. Synthesis and Application of a Novel Metal–Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater. Polymers. 2023; 15(9):2150. https://doi.org/10.3390/polym15092150
Chicago/Turabian StyleYu, Li, Tu Lan, Guoyuan Yuan, Chongxiong Duan, Xiaoqin Pu, and Ning Liu. 2023. "Synthesis and Application of a Novel Metal–Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater" Polymers 15, no. 9: 2150. https://doi.org/10.3390/polym15092150