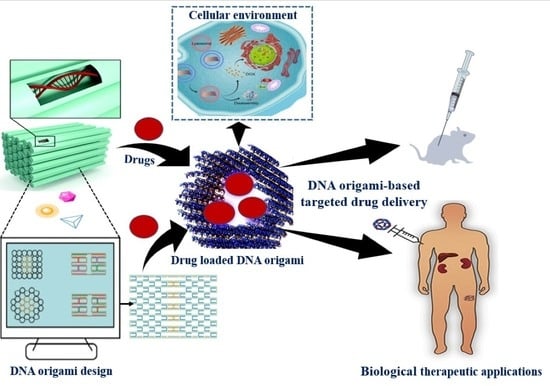
Unravelling the Drug Encapsulation Ability of Functional DNA Origami Nanostructures: Current Understanding and Future Prospects on Targeted Drug Delivery
Abstract
:1. Introduction
2. Overview and Structural Features of DNA Origami Nanostructures
3. Synthesis and Assembly of DNA Origami Nanostructures
4. DNA-Origami-Based Approaches and Therapeutic Strategies for Targeted Drug Delivery
5. Cellular Targeting and Entry of Drug Encapsulated DNA Origami
6. Challenges and Future Perspectives of DNA Origami Nanostructures as Delivery System
6.1. Challenges
6.2. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, S.; Tatini, R. Folate-Functionalized DNA Origami for Targeted Delivery of Doxorubicin to Triple-Negative Breast Cancer. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sleiman, H.F. DNA Nanotechnology. Nat. Rev. Mater. 2017, 3, 17068. [Google Scholar] [CrossRef]
- Machtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Palaria, B.; Tiwari, V.; Tiwari, A.; Aslam, R.; Kumar, A.; Sahoo, B.M.; Kumar, M.; Singh, S.; Kumar, S. Nanostructured Lipid Carriers: A Promising Carrier in Targeted Drug Delivery System. Curr. Nanomat. 2023, 8, 23–43. [Google Scholar]
- Khatir, N.M.; Abdul-Malek, Z.; Banihashemian, S.M. Influences of magnetic fields on current–voltage characteristics of gold-DNA-gold structure with variable gaps. Mater. Sci. Semicond. Process. 2015, 36, 134–139. [Google Scholar] [CrossRef]
- Khatir, N.M.; Sabbagh, F. Green Facile Synthesis of Silver-Doped Zinc Oxide Nanoparticles and Evaluation of Their Effect on Drug Release. Materials 2022, 15, 5536. [Google Scholar] [CrossRef]
- Khatir, N.M.; Abdul-Malek, Z.; Zak, A.K.; Akbari, A.; Sabbagh, F. Sol–gel grown Fe-doped ZnO nanoparticles: Antibacterial and structural behaviors. J. Sol-Gel Sci. Technol. 2016, 78, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Weiden, J.; Bastings, M.M. DNA origami nanostructures for controlled therapeutic drug delivery. Curr. Opin. Colloid Interface Sci. 2021, 52, 101411. [Google Scholar] [CrossRef]
- Schneider, F.; Möritz, N.; Dietz, H. The sequence of events during folding of a DNA origami. Sci Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Andersen, E.S.; Dong, M.; Nielsen, M.M. DNA origami design of dolphin-shaped structures with flexible tails. ACS Nano 2008, 2, 1213–1218. [Google Scholar] [CrossRef]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into Nanoscale Three-Dimensional Shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Jun, H.; Wang, X.; Bricker, W.P.; Bathe, M. Automated sequence design of 2D wireframe DNA origami with honeycomb edges. Nat. Commun. 2019, 10, 5419. [Google Scholar] [CrossRef] [Green Version]
- Benson, E.; Mohammed, A.; Gardell, J. DNA rendering of polyhedral meshes at the nanoscale. Nature 2015, 523, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Dietz, H.; Douglas, S.M.; Shih, W.M. Folding DNA into twisted and curved nanoscale shapes. Science 2009, 325, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Wagenbauer, K.F.; Sigl, C.; Dietz, H. Gigadalton-scale shape-programmable DNA assemblies. Nature 2017, 552, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Qi, X.; Myhrvold, C. Single-stranded DNA and RNA origami. Science 2017, 358, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerling, T.; Wagenbauer, K.F.; Neuner, A.M.; Dietz, H. Dynamic DNA devices and assemblies formed by shape-complementary, non-base pairing 3D components. Science 2015, 347, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Gu, H.; Li, Q.; Fan, C. Concept and Development of Framework Nucleic Acids. J. Am. Chem. Soc. 2018, 140, 17808–17819. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Jing, X. Complex silica composite nanomaterials templated with DNA origami. Nature 2018, 559, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Fan, C.; Gothelf, K.V.; Li, J.; Lin, C.; Liu, L.; Liu, N.; Nijenhuis, M.A.; Saccà, B.; Simmel, F.C.; et al. DNA origami. Nat. Rev. Met. Prim. 2021, 1, 13. [Google Scholar] [CrossRef]
- Liu, N.; Liedl, T. DNA-Assembled Advanced Plasmonic Architectures. Chem. Rev. 2018, 118, 3032–3053. [Google Scholar] [CrossRef] [PubMed]
- Yurke, B.; Turberfield, A.J.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangreave, J.; Han, D.; Liu, Y.; Yan, H. DNA origami: A history and current perspective. Curr. Opin. Chem. Biol. 2010, 14, 608–615. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef]
- Baig, M.M.F.A.; Xia, X.H. The PA-receptor mediated internalization of carboplatin loaded poly-anionic DNA-nanowires for effective treatment of resistant hepatic-cancer HepG-2 cells. Appl. Nanosci. 2020, 10, 1915–1926. [Google Scholar] [CrossRef]
- Mariconti, M. DNA-Protein Nanogels as a New Class of Tunable Nanobiomaterials: From Enzymatic Nanoreactors to Transfection of Active Proteins. Ph.D. Thesis, Université Paris sciences et Lettres, Paris, France, 2021. [Google Scholar]
- Wang, D.X.; Wang, J.; Wang, Y.X.; Du, Y.C.; Huang, Y.; Tang, A.N.; Cui, Y.X.; Kong, D.M. DNA nanostructure-based nucleic acid probes: Construction and biological applications. Chem. Sci. 2021, 12, 7602–7622. [Google Scholar] [CrossRef]
- Adamczyk, A.K.; Huijben, T.A.; Sison, M.; Di Luca, A.; Chiarelli, G.; Vanni, S.; Brasselet, S.; Mortensen, K.I.; Stefani, F.D.; Pilo-Pais, M.; et al. DNA self-assembly of single molecules with deterministic position and orientation. ACS Nano 2022, 16, 16924–16931. [Google Scholar] [CrossRef]
- Ijäs, H.; Shen, B.; Heuer-Jungemann, A.; Keller, A.; Kostiainen, M.A.; Liedl, T.; Ihalainen, J.A.; Linko, V. Unraveling the interaction between doxorubicin and DNA origami nanostructures for customizable chemotherapeutic drug release. Nucleic Acids Res. 2021, 49, 3048–3062. [Google Scholar] [CrossRef]
- Halley, P.D.; Lucas, C.R.; McWilliams, E.M. Daunorubicin-Loaded DNA Origami Nanostructures Circumvent Drug-Resistance Mechanisms in a Leukemia Model. Small 2016, 12, 308–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, X.; Ma, X.; Xue, X. A Photosensitizer-Loaded DNA Origami Nanosystem for Photodynamic Therapy. ACS Nano 2016, 10, 3486–3495. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Liu, J.; Liu, M. A Nanobody-Conjugated DNA Nanoplatform for Targeted Platinum-Drug Delivery. Angew Chem. Int. Ed. Engl. 2019, 58, 14224–14228. [Google Scholar] [CrossRef]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.L.; Fleury Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef] [PubMed]
- Thurston, D.E.; Pysz, I. Chemistry and Pharmacology of Anticancer Drugs; CRC Press: Boca Raton, FL, USA, 2021; Volume 89, pp. 323–330. [Google Scholar]
- Bu, Y.Z.; Xu, J.R.; Luo, Q.; Chen, M.; Mu, L.M.; Lu, W.L. A precise nanostructure of folate-overhung mitoxantrone dna tetrahedron for targeted capture leukemia. Nanomaterials 2020, 10, 951. [Google Scholar] [CrossRef]
- Sala, L.; Perecko, T.; Mestek, O.; Pinkas, D.; Homola, T.; Kocisek, J. Cisplatin-Cross-Linked DNA Origami Nanostructures for Drug Delivery Applications. ACS Appl. Nano Mater. 2022, 5, 13267–13275. [Google Scholar] [CrossRef]
- Nathiya, S.; Durga, M.; Thiyagarajan, D. Quercetin, encapsulated quercetin and its application—A review. Int. J. Pharm. Pharm. Sci. 2014, 32, 20–26. [Google Scholar]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Koba, M.; Konopa, J. Actinomycin D and its mechanisms of action. Postepy Hig. Med. Dosw. 2005, 59, 290–298. [Google Scholar]
- Mei, Q.; Wei, X.; Su, F. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 2011, 11, 1477–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, C.J.; Lucas, C.R.; O’Brien, F.J.; Castro, C.E. DNA origami: Folded DNA-nanodevices that can direct and interpret cell behavior. Adv. Mater. 2016, 28, 5509–5524. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; An, M.; Jones, E.; Liu, H. Targeting Suppressive Oligonucleotide to Lymph Nodes Inhibits Toll-like Recep-tor-9-Mediated Activation of Adaptive Immunity. Pharm. Res. 2018, 35, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pei, H.; Zhu, B. Self-assembled multivalent DNA nanostructures for noninvasive intracellular delivery of immunostimulatory CpG oligonucleotides. ACS Nano 2011, 5, 8783–8789. [Google Scholar] [CrossRef]
- Huang, E.; Showalter, L.; Xu, S.; Czernliecki, B.J.; Koski, G.K. Calcium mobilizing treatment acts as a co-signal for TLR-mediated induction of Interleukin-12 (IL-12p70) secretion by murine bone marrow-derived dendritic cells. Cell Immunol. 2017, 314, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Yang, Z.; Xu, K.; Wang, C.; Liang, H. DNA nanostructure as an efficient drug delivery platform for immunotherapy. Front. Pharmacol. 2020, 10, 1585–1589. [Google Scholar] [CrossRef]
- Xu, T.; Yu, S.; Sun, Y. DNA Origami Frameworks Enabled Self-Protective siRNA Delivery for Dual Enhancement of Chemo-Photothermal Combination Therapy. Small 2021, 17, 210–215. [Google Scholar] [CrossRef]
- Zeng, Y.C.; Young, O.J.; Wintersinger, C.M.; Anastassacos, F.M.; MacDonald, J.I.; Isinelli, G.; Dellacherie, M.O.; Sobral, M.; Bai, H.; Graveline, A.R.; et al. Optimizing CpG spatial distribution with DNA origami for Th1-polarized therapeutic vaccination. BioRxiv 2022. [Google Scholar] [CrossRef]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Jiang, Q.; Shi, Y.; Zhang, Q. A Self-Assembled DNA Origami-Gold Nanorod Complex for Cancer Theranostics. Small 2015, 11, 5134–5141. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Q.; Beziere, N. DNA-Nanostructure-Gold-Nanorod Hybrids for Enhanced In Vivo Optoacoustic Imaging and Photothermal Therapy. Adv. Mater. 2016, 28, 10000–10007. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Jiang, Q.; Sun, J. Programming DNA Nanoassembly for Enhanced Photodynamic Therapy. Angew Chem. Int. Ed. Engl. 2020, 59, 1897–1905. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, S.; Liu, J. Rationally designed DNA-origami nanomaterials for drug delivery in vivo. Adv. Mater. 2018, 31, 1804785. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiang, W.; Fan, Q.; Wang, L.; Chao, J. A DNA origami nanostructure embedded with NQO1-activated prodrugs for precision drug delivery. Chem. Comm. 2023, 59, 912–915. [Google Scholar] [CrossRef]
- Andreas, W.; Sebastian, L. 3D DNA origami nanoparticles: From basic design principles to emerging applications in soft matter and (bio) nanosciences. Angew. Chem. Int. Ed. 2018, 57, 10436–10448. [Google Scholar]
- Mishra, S.; Feng, Y.; Endo, M.; Sugiyama, H. Advances in DNA origami–cell interfaces. ChemBioChem 2020, 21, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, L.; Liu, S.; Zhao, S.; Jiang, Q.; Ding, B. A tailored DNA nanoplatform for synergistic RNAi-/chemotherapy of multidrug-resistant tumors. Angew. Chem. Int. 2018, 57, 15486–15490. [Google Scholar] [CrossRef]
- Liu, K.; Xu, C.; Liu, J. Regulation of cell binding and entry by DNA origami mediated spatial distribution of aptamers. J. Mater. Chem. B. 2020, 8, 6802–6809. [Google Scholar] [CrossRef]
- Palazzolo, S.; Hadla, M.; Spena, C.R.; Bayda, S.; Kumar, V.; Re, F.L. Proof-of-concept multistage biomimetic liposomal DNA origami nanosystem for the remote loading of doxorubicin. ACS Med. Chem. Lett. 2019, 10, 517–521. [Google Scholar] [CrossRef]
- Pan, Q.; Nie, C.; Hu, Y.; Yi, J.; Liu, C.; Zhang, J. Aptamer-functionalized DNA origami for targeted codelivery of antisense oligonucleotides and doxorubicin to enhance therapy in drug-resistant cancer cells. ACS Appl. Mater. Interfaces. 2019, 12, 400–409. [Google Scholar] [CrossRef]
- Song, L.; Jiang, Q.; Liu, J.; Li, N. DNA origami/gold nanorods hybrid nanostructures for the circumvention of drug resistance. Nanoscale 2017, 9, 7750–7754. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Krishnan, S.; Simmel, F.C. Electrotransfection of Polyamine Folded DNA Origami Structures. Nano Lett. 2016, 16, 6683–6690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zheng, J.; Song, E. Self-assembled, aptamer-tethered DNA nanotrains for targeted transport of molecular drugs in cancer theranostics. Proc. Natl. Acad. Sci. USA 2013, 110, 7998–8003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Yang, J.; Zhan, P. Self-Assembled DNA Dendrimer Nanoparticle for Efficient Delivery of Immunostimulatory CpG Motifs. ACS Appl. Mater. Interfaces 2017, 9, 20324–20329. [Google Scholar] [CrossRef]
- Schüller, V.J.; Heidegger, S.; Sandholzer, N. Cellular immunostimulation by CpG-sequence-coated DNA origami structures. ACS Nano 2011, 5, 9696–9702. [Google Scholar] [CrossRef] [Green Version]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control Release 2018, 274, 24–34. [Google Scholar] [CrossRef]
- Liu, J.; Song, L.; Liu, S.; Jiang, Q.; Liu, Q. A DNA-based nanocarrier for efficient gene delivery and combined cancer therapy. Nano Lett. 2018, 18, 3328–3334. [Google Scholar] [CrossRef]
- Ijas, H.; Hakaste, I.; Shen, B.; Kostiainen, M.A.; Linko, V. Reconfigurable DNA origami nanocapsule for pH-controlled encapsulation and display of cargo. ACS Nano 2019, 13, 5959–5967. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.R.; Lamarre, B.; Pyne, A.L.; Noble, J.E.; Ryadnov, M.G. DNA origami inside-out viruses. ACS Synth. Biol. 2018, 7, 767–773. [Google Scholar] [CrossRef]
- Ora, A.; Järvihaavisto, E.; Zhang, H.; Auvinen, H.; Santos, A.; Kostiainen, M.A. Cellular delivery of enzyme-loaded DNA origami. Chem. Commun. 2016, 52, 14161–14164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Duan, F.; Liu, S.; Wu, T.; Shang, Y.; Tian, R. Efficient intracellular delivery of RNase A using DNA origami carriers. ACS Appl. Mater. Interfaces 2019, 11, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, F.; Ramakrishnan, S.; Shen, B.; Grundmeier, G.; Kostiainen, M.A.; Linko, V. Superstructure-dependent loading of DNA origami nanostructures with a groove-binding drug. ACS Omega 2018, 3, 9441–9448. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.-X.; Shaw, A.; Zeng, X.; Benson, E.; Nyström, A.M.; Hogberg, B. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano 2012, 6, 8684–8691. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Zhang, H.; Qu, X.; Zhang, X.; Chen, D.; Ding, R. Gold nanorods, DNA origami, and porous silicon nanoparticle-functionalized biocompatible double emulsion for versatile targeted therapeutics and antibody combination therapy. Adv. Mater. 2016, 28, 10195–10203. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Sun, Y.; Xiao, M.; Li, L.; Liu, X.; Jin, H. Multivalent aptamer-modified DNA origami as drug delivery system for targeted cancer therapy. Chem. Res. Chin. Univ. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Schaffert, D.H.; Okholm, A.H.; Sørensen, R.S.; Nielsen, J.S.; Tørring, T.; Rosen, C.B. Intracellular delivery of a planar DNA origami structure by the transferrin-receptor internalization pathway. Small 2016, 12, 2634–2640. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, L.; Wu, G.; Li, J.; Sun, Y.; Hou, Y. DNA origami-enabled engineering of ligand-drug conjugates for targeted drug delivery. Small 2020, 16, 1904857. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, P.; Zhao, Z.; Wang, D.; Nannapaneni, S.; Zhang, C. Systemic delivery of bc12-targeting siRNA by DNA nanoparticles suppresses cancer cell growth. Angew. Chem. Int. Ed. 2017, 56, 16023–16027. [Google Scholar] [CrossRef]
- Zeng, Y.; Nixon, R.L.; Liu, W.; Wang, R. The applications of functionalized DNA nanostructures in bioimaging and cancer therapy. Biomaterials 2021, 268, 120560. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Wu, X.; Ding, B. Multifunctional DNA origami nanoplatforms for drug delivery. Chem. Asian J. 2019, 14, 2193–2202. [Google Scholar] [CrossRef]
- Jorge, F.; Aviñó, A.; Pais, A.A.; Eritja, R.; F‘abrega, C. DNA-based nanoscaffolds as vehicles for 5-fluoro-2′ -deoxyuridine oligomers in colorectal cancer therapy. Nanoscale 2018, 10, 7238–7249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Benson, E.; Fördős, F.; Lolaico, M.; Baars, I.; Fang, T.; Teixeira, A.I.; Högberg, B. DNA origami penetration in cell spheroid tissue models is enhanced by wireframe design. Adv. Mat. 2021, 33, 2008457. [Google Scholar] [CrossRef]
- Chao, J.; Liu, H.; Su, S.; Wang, L.; Huang, W.; Fan, C. Structural DNA nanotechnology for intelligent drug delivery. Small 2014, 10, 4626–4635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, D.X.; Liu, B.; Jing, X.; Chen, D.Y.; Tang, A.N.; Cui, Y.X.; Kong, D.M. Recent Advances in Constructing Higher-Order DNA Structures. Chem. Asian J. 2022, 17, 202101315. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, J.; Yin, P.; Voigt, C.A. Genetic encoding of DNA nanostructures and their self-assembly in living bacteria. Nat. Commun. 2016, 7, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, R.; Ratanalert, S.; Zhang, K. Designer nanoscale DNA assemblies programmed from the top down. Science 2016, 352, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Hahn, J.; Wickham, S.F.; Shih, W.M.; Perrault, S.D. Addressing the instability of DNA nanostructures in tissue culture. ACS Nano 2014, 8, 8765–8775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Meyer, T.A.; Pan, V.; Dutta, P.K.; Ke, Y. The beauty and utility of DNA origami. Chem 2017, 2, 359–382. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart drug delivery nanocarriers with self-assembled DNA nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, J.; Dhakal, S. Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat. Commun. 2016, 7, 10619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Hu, C.; Wang, P. Growth and origami folding of DNA on nanoparticles for high-efficiency molecular transport in cellular imaging and drug delivery. Angew Chem. Int. Ed. Engl. 2015, 54, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Song, C.; Nangreave, J.; Liu, X.; Lin, L.; Qiu, D. DNA origami as a carrier for circumvention of drug resistance. J. Am. Chem. Soc. 2012, 134, 13396–13403. [Google Scholar] [CrossRef] [PubMed]





| Shapes of DNA Origami Nanostructures | Diameter | Length | Therapeutic Agent/Drug Loaded | Cell Lines | Targeting Ligands | Targeted Sites or Triggered Conditions | Target Model | Ref. |
|---|---|---|---|---|---|---|---|---|
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | BMEPC | MCF-7 | ------- | Photodynamic therapy (PDT) | In vitro | [58] |
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | Gold nanorods | 4T1-fLuc | ------- | Photodynamic therapy | In vitro | [58] |
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | DOX,Gold nanorods. | MCF-7/ADR | ------- | Photothermal therapy/MUC1 | Orthotopic transplantation | [53] |
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | Therapeutic gene p53, DOX | MCF-7R, MCF-7 | MUC1 aptamers | MUC1/genes | Subcutaneous xenograft | [59] |
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | hairpin RNA, DOX | MCF-7R, MCF-7 | MUC1 aptamers | MUC1/genes | In vitro | [52] |
| Triangle-shaped DNA origami | 12.5 nm. | 67 nm. | DOX | HeLa | Sgc8 aptamers | PTK7 | In vitro | [60] |
| Tube-shaped DNA origami | 2.25 nm. | 0.34 nm. | DOX | MDA-MB-231, MDA-MB-468, MCF-7 | ------- | ------- | In vitro | [61] |
| Tube-shaped DNA origami | 2.25 nm. | 0.34 nm. | DOX | LNCaP (PSMA+), PC-3 | DUPA | PMSA | In vitro | [24] |
| Rectangle-shaped DNA origami | 70 nm. | 2 nm. | Antisense oligonucleotides | HeLa/ADR, MCF-7/ADR | MUC1 aptamers | mRNA of B-cell protein and P-glycoprotein | In vitro | [62] |
| Rectangle-shaped DNA origami | 70 nm. | 2 nm. | 5-fluoro-2′ -deoxyuridine | HTB-38, HCC-2998 | ------- | ------- | In vitro | [27] |
| Tube-, triangle-shaped DNA origami | Tube—2.25 nm. Triangle—12.5 nm. | Tube—0.34 nm. Triangle—67 nm. | Gold nanorods | MCF-7 | ------- | Photothermal therapy | Subcutaneous xenograft | [63] |
| Tube-, triangle-shaped DNA origami | Tube—2.25 nm. Triangle—12.5 nm. | Tube—0.34 nm. Triangle—67 nm. | DOX | MCF-7R | ------- | ------- | In vitro | [34] |
| Rectangle-, tube-shaped DNA origami | Rectangle—70 nm. Tube—2.25 nm. | Rectangle—2 nm. Tube—0.34 nm. | Thrombin | HUVECs, MDA-MB-231, SK-OV3, B16-F10, bEnd.3 | AS1411 aptamers | Nucleolin | In vitro | [62] |
| Rectangle-, tube-shaped DNA origami | Rectangle- 70 nm. Tube—2.25 nm. | Rectangle- 2 nm. Tube—0.34 nm. | siRNA | DMS53, H1299 | ------- | mRNA of BCL-2 | In vitro | [34] |
| Tube-, rectangle-, triangle-shaped DNA origami | Tube—2.25 nm. Rectangle—70 nm. Triangle—12.5 nm. | Tube—0.34 nm. Rectangle—2 nm. Triangle—67 nm. | DOX | MDA-MB-231 | ------- | ------- | In vitro | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosal, S.; Bag, S.; Bhowmik, S. Unravelling the Drug Encapsulation Ability of Functional DNA Origami Nanostructures: Current Understanding and Future Prospects on Targeted Drug Delivery. Polymers 2023, 15, 1850. https://doi.org/10.3390/polym15081850
Ghosal S, Bag S, Bhowmik S. Unravelling the Drug Encapsulation Ability of Functional DNA Origami Nanostructures: Current Understanding and Future Prospects on Targeted Drug Delivery. Polymers. 2023; 15(8):1850. https://doi.org/10.3390/polym15081850
Chicago/Turabian StyleGhosal, Souvik, Sagar Bag, and Sudipta Bhowmik. 2023. "Unravelling the Drug Encapsulation Ability of Functional DNA Origami Nanostructures: Current Understanding and Future Prospects on Targeted Drug Delivery" Polymers 15, no. 8: 1850. https://doi.org/10.3390/polym15081850