Apical Sealing and Bioactivity of an Experimental Gutta-Percha Containing Niobium Phosphate Bioglass
Abstract
:1. Introduction
2. Materials and Methods
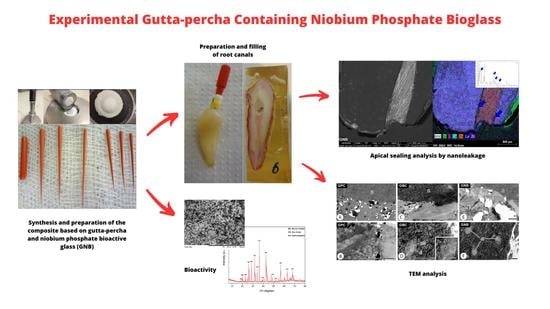
2.1. Synthesis and Preparation of the Composite Based on Gutta-Percha and Niobium Phosphate Bioactive Glass (GNB)
2.2. Preparation of Simulated Body Fluid (SBF)
2.3. Preparation and Filling of Root Canals
2.4. Apical Sealing Analysis by Nanoleakage
2.5. Transmission Electron Microscopy (TEM)
2.6. Bioactivity Analysis
2.7. Statistical Analysis
3. Results
3.1. Lanthanum Nanoleakage
3.2. TEM Analysis
3.3. Bioactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sjogren, U.; Hagglund, B.; Sundqvist, G.; Wing, K. Factors Affecting the Long-Term Results of Endodontic Treatment. J. Endod. 1990, 16, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.M.; Duque, C. Métodos de avaliação da resistência à infiltração em obturações endodônticas. Rev. Bras. Odontol. 2012, 69, 34–38. [Google Scholar]
- Habl, C.; Bodenwinkler, A.; Stürzlinger, H. Endodontic Treatment of Molars. GMS Health Technol. Assess. 2006, 2, Doc03. [Google Scholar]
- Lima, N.F.F.; dos Santos, P.R.N.; da Silva Pedrosa, M.; Delboni, M.G. Cimentos biocerâmicos em endodontia: Revisão de literatura. Rev. Fac. Odontol.—UPF 2017, 22, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, K.K.; Araujo, T.P.; Carvalho, E.M.; Meier, M.M.; Tanaka, A.; Carvalho, C.N.; Bauer, J. Bioactivity and Properties of an Adhesive System Functionalized with an Experimental Niobium-Based Glass. J. Mech. Behav. Biomed. Mater. 2018, 78, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of Composites Containing Bioactive Glasses on Demineralized Dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Kulbacka, J.; Nowakowska-Toporowska, A. Mechanical Properties, Cytotoxicity, and Fluoride Ion Release Capacity of Bioactive Glass-Modified Methacrylate Resin Used in Three-Dimensional Printing Technology. Materials 2022, 15, 1133. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [Green Version]
- Staffoli, S.; Plotino, G.; Nunez Torrijos, B.G.; Grande, N.M.; Bossù, M.; Gambarini, G.; Polimeni, A. Regenerative Endodontic Procedures Using Contemporary Endodontic Materials. Materials 2019, 12, 908. [Google Scholar] [CrossRef] [Green Version]
- Alani, A.; Knowles, J.C.; Chrzanowski, W.; Ng, Y.-L.; Gulabivala, K. Ion Release Characteristics, Precipitate Formation and Sealing Ability of a Phosphate Glass-Polycaprolactone-Based Composite for Use as a Root Canal Obturation Material. Dent. Mater. 2009, 25, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Mohn, D.; Bruhin, C.; Luechinger, N.A.; Stark, W.J.; Imfeld, T.; Zehnder, M. Composites Made of Flame-Sprayed Bioactive Glass 45S5 and Polymers: Bioactivity and Immediate Sealing Properties. Int. Endod. J. 2010, 43, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Mohn, D.; Zehnder, M.; Imfeld, T.; Stark, W.J. Radio-Opaque Nanosized Bioactive Glass for Potential Root Canal Application: Evaluation of Radiopacity, Bioactivity and Alkaline Capacity. Int. Endod. J. 2010, 43, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Marending, M.; Bubenhofer, S.B.; Sener, B.; De-Deus, G. Primary Assessment of a Self-Adhesive Gutta-Percha Material. Int. Endod. J. 2013, 46, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.N.; Bauer, J.; Ferrari, P.H.P.; Souza, S.F.C.; Soares, S.P.; Loguercio, A.D.; Bombana, A.C. Influence of Calcium Hydroxide Intracanal Medication on Bond Strength of Two Endodontic Resin-Based Sealers Assessed by Micropush-out Test. Dent. Traumatol. 2013, 29, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Wong, F.S.L.; Johal, A.; Hill, R.G. Fluoride Containing Bioactive Glass Composite for Orthodontic Adhesives—Ion Release Properties. Dent. Mater. 2017, 33, 1324–1329. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a Dentin Bonding Resin to Become Bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Azizabadi, N.; Azar, P.A.; Tehrani, M.S.; Derakhshi, P. Synthesis and Characteristics of Gel-Derived SiO2-CaO-P2O5-SrO-Ag2O-ZnO Bioactive Glass: Bioactivity, Biocompatibility, and Antibacterial Properties. J. Non-Cryst. Solids 2021, 556, 120568. [Google Scholar] [CrossRef]
- Baino, F. Bioactive Glasses—When Glass Science and Technology Meet Regenerative Medicine. Ceram. Int. 2018, 44, 14953–14966. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Martinelli, J.R.; Bauer, J.; Haapasalo, M.; Shen, Y.; Bradaschia-Correa, V.; Manso, A.P.; Gavini, G. Micropush-out Dentine Bond Strength of a New Gutta-Percha and Niobium Phosphate Glass Composite. Int. Endod. J. 2015, 48, 451–459. [Google Scholar] [CrossRef]
- Altmann, A.S.P.; Collares, F.M.; Balbinot, G.D.S.; Leitune, V.C.B.; Takimi, A.S.; Samuel, S.M.W. Niobium Pentoxide Phosphate Invert Glass as a Mineralizing Agent in an Experimental Orthodontic Adhesive. Angle Orthod. 2017, 87, 759–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, C.C.B.; Olivi, L.T.; Carvalho, C.N.; Gavini, G.; Sipert, C.R. Cytotoxic Effect of Niobium Phosphate Glass-Based Gutta-Percha Points on Periodontal Ligament Fibroblasts In Vitro. J. Endod. 2020, 46, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Onay, E.O.; Ungor, M.; Unver, S.; Ari, H.; Belli, S. An In Vitro Evaluation of the Apical Sealing Ability of New Polymeric Endodontic Filling Systems. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e49–e54. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Kokate, S.; Hegde, V. Comparative Assessment of Apical Sealing Ability of Three Different Endodontic Sealers: A Scanning Electron Microscopic Study. J. Pierre Fauchard Acad. 2014, 28, 78–82. [Google Scholar] [CrossRef]
- Hamdan, R.; Michetti, J.; Dionnet, C.; Diemer, F.; Georgelin-Gurgel, M. In-Vitro Evaluation of Apical Microleakage of Two Obturation Methods of Immature Permanent Teeth: Orthograde Apical Plug of Mineral Trioxide Aggregate and Root Canal Filling Combining Custom Gutta-Percha Cone with Calcium Silicate-Based Sealer. G. Ital. Endod. 2017, 31, 89–95. [Google Scholar] [CrossRef]
- Going, R.E. Microleakage around Dental Restorations: A Summarizing Review. J. Am. Dent. Assoc. 1972, 84, 1349–1357. [Google Scholar] [CrossRef]
- Modaresi, J.; Baharizade, M.; Shareghi, A.; Ahmadi, M.; Daneshkazemi, A. Copper Ion as a New Leakage Tracer. J. Dent. 2013, 14, 155–159. [Google Scholar]
- Shaklai, M.; Tavassoli, M. Lanthanum as an Electron Microscopic Stain. J. Histochem. Cytochem. 1982, 30, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Squier, C.; Edie, J. Localization of Lanthanum Tracer in Oral Epithelium Using Transmission Electron Microscopy and the Electron Microprobe. Histochem. J. 1983, 15, 1123–1130. [Google Scholar] [CrossRef]
- MacKenzie, M.L.; Ghabriel, M.N.; Allt, G. The Blood-Nerve Barrier: An In Vivo Lanthanum Tracer Study. J. Anat. 1987, 154, 27–37. [Google Scholar]
- ISO 23317:2012; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Standard Organization: Geneva, Switzerland, 2012.
- Soares, A.M.; Arana-Chavez, V.E.; Reid, A.R.; Katchburian, E. Lanthanum Tracer and Freeze-Fracture Studies Suggest that Compartmentalisation of Early Bone Matrix May Be Related to Initial Mineralisation. J. Anat. 1992, 181, 345–356. [Google Scholar] [PubMed]
- Siqueira, J.F., Jr.; Rôças, I.N.; Lopes, H.P.; Alves, F.R.F.; Oliveira, J.C.M.; Armada, L.; Provenzano, J.C. Princípios biológicos do tratamento endodôntico de dentes com polpa necrosada e lesão perirradicular. Rev. Bras. Odontol. 2012, 69, 8–14. [Google Scholar]
- Tabassum, S.; Khan, F.R. Failure of Endodontic Treatment: The Usual Suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Amarante de Camargo, D.A.; Sinhoreti, M.A.C.; Correr-Sobrinho, L.; de Sousa Neto, M.D.; Consani, S. Influence of the Methodology and Evaluation Criteria on Determining Microleakage in Dentin-Restorative Interfaces. Clin. Oral Investig. 2006, 10, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Youngson, C.C.; Jones, J.C.; Manogue, M.; Smith, I.S. In Vitro Dentinal Penetration by Tracers Used in Microleakage Studies. Int. Endod. J. 1998, 31, 90–99. [Google Scholar] [CrossRef]
- Tay, F.R.; King, N.M.; Chan, K.; Pashley, D.H. How Can Nanoleakage Occur in Self-Etching Adhesive Systems that Demineralize and Infiltrate Simultaneously? J. Adhes. Dent. 2002, 4, 255–269. [Google Scholar] [PubMed]
- Takano, Y.; Crenshaw, M.A. The Penetration of Intravascularly Perfused Lanthanum into the Ameloblast Layer of Developing Rat Molar Teeth. Arch. Oral Biol. 1980, 25, 505–511. [Google Scholar] [CrossRef]
- Chevalier, L.; Selim, J.; Genty, D.; Baste, J.M.; Piton, N.; Boukhalfa, I.; Hamzaoui, M.; Pareige, P.; Richard, V. Electron Microscopy Approach for the Visualization of the Epithelial and Endothelial Glycocalyx. Morphologie 2017, 101, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Torres-Quintana, M.A.; Septier, D.; Goldberg, M. Differences in the Pattern of Lanthanum Diffusion into Predentine and Dentine in Mouse Incisors and Molars. Arch. Oral Biol. 1999, 44, 351–360. [Google Scholar] [CrossRef]
- Patil, P.; Rathore, V.P.S.; Hotkar, C.; Savgave, S.S.; Raghavendra, K.; Ingale, P. A Comparison of Apical Sealing Ability between GuttaFlow and AH plus: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2016, 6, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Orhan, K.; Celikten, B.; Orhan, A.I.; Tufenkci, P.; Sevimay, S. Evaluation of the Sealing Ability of Different Root Canal Sealers: A Combined SEM and Micro-CT Study. J. Appl. Oral Sci. 2018, 26, e20160584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paqué, F.; Sirtes, G. Apical Sealing Ability of Resilon/Epiphany versus Gutta-Percha/AH Plus: Immediate and 16-Months Leakage. Int. Endod. J. 2007, 40, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Wesselink, P.R. Endodontic Leakage Studies Reconsidered. Part I. Methodology, Application and Relevance. Int. Endod. J. 1993, 26, 37–43. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Grazziotin-Soares, R.; de Miranda Candeiro, G.T.; Gallego Martinez, L.; de Souza, J.P.; Santos Oliveira, P.; Bauer, J.; Gavini, G. Micro Push-out Bond Strength and Bioactivity Analysis of a Bioceramic Root Canal Sealer. Iran. Endod. J. 2017, 12, 343–348. [Google Scholar] [CrossRef]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate Glasses for Tissue Engineering: Part 1. Processing and Characterisation of a Ternary-Based P2O5-CaO-Na2O Glass System. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Magalhães, A.; Mazali, I.O.; Bertran, C.A. Effect of Niobium Oxide on the Structure and Properties of Melt-Derived Bioactive Glasses. J. Am. Ceram. Soc. 2014, 97, 3843–3852. [Google Scholar] [CrossRef]
- Sene, F.F.; Martinelli, J.R.; Gomes, L. Synthesis and Characterization of Niobium Phosphate Glasses Containing Barium and Potassium. J. Non-Cryst. Solids 2004, 348, 30–37. [Google Scholar] [CrossRef]
- Godley, R.; Starosvetsky, D.; Gotman, I. Bonelike Apatite Formation on Niobium Metal Treated in Aqueous NaOH. J. Mater. Sci. Mater. Med. 2004, 15, 1073–1077. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kim, H.-M.; Kokubo, T.; Ohtsuki, C.; Nakamura, T. Apatite-Forming Ability of Niobium Oxide Gels in a Simulated Body Fluid. J. Ceram. Soc. Jpn. 2001, 109, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Roggendorf, M.J.; Ebert, J.; Petschelt, A.; Frankenberger, R. Influence of Moisture on the Apical Seal of Root Canal Fillings with Five Different Types of Sealer. J. Endod. 2007, 33, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Zmener, O.; Pameijer, C.H.; Serrano, S.A.; Vidueira, M.; Macchi, R.L. Significance of Moist Root Canal Dentin with the Use of Methacrylate-Based Endodontic Sealers: An In Vitro Coronal Dye Leakage Study. J. Endod. 2008, 34, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Nagas, E.; Uyanik, M.O.; Eymirli, A.; Cehreli, Z.C.; Vallittu, P.K.; Lassila, L.V.J.; Durmaz, V. Dentin Moisture Conditions Affect the Adhesion of Root Canal Sealers. J. Endod. 2012, 38, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Gritti, G.C.; Cavalcante, S.I.A.; Maia-Filho, E.M.; Bauer, J.; Bandéca, M.C.; Gavini, G.; Carvalho, C.N. Effect of Rewetting Solutions on Micropush-out Dentin Bond Strength of New Bioceramic Endodontic Material. Braz. Oral Res. 2017, 31, e76. [Google Scholar] [CrossRef] [Green Version]






| Groups | Nanoleakage | p-Value | |
|---|---|---|---|
| Absent | Present | ||
| GPC | 8 | 1 | p = 0.13 * |
| GBC | 9 | 0 | |
| GNB | 6 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, R.F.; Carvalho, C.N.; Bradaschia-Correa, V.; Gonçalves, B.L.L.; Arana-Chavez, V.; Carvalho, A.P.L.d.; Nogueira, A.P.A.; Grazziotin-Soares, R.; Bauer, J.; Gavini, G.; et al. Apical Sealing and Bioactivity of an Experimental Gutta-Percha Containing Niobium Phosphate Bioglass. Polymers 2023, 15, 1679. https://doi.org/10.3390/polym15071679
Sampaio RF, Carvalho CN, Bradaschia-Correa V, Gonçalves BLL, Arana-Chavez V, Carvalho APLd, Nogueira APA, Grazziotin-Soares R, Bauer J, Gavini G, et al. Apical Sealing and Bioactivity of an Experimental Gutta-Percha Containing Niobium Phosphate Bioglass. Polymers. 2023; 15(7):1679. https://doi.org/10.3390/polym15071679
Chicago/Turabian StyleSampaio, Ruan Ferreira, Ceci Nunes Carvalho, Vivian Bradaschia-Correa, Bruna Laís Lins Gonçalves, Victor Arana-Chavez, Alexandre P. Lima de Carvalho, Amanda Palmeira Arruda Nogueira, Renata Grazziotin-Soares, José Bauer, Giulio Gavini, and et al. 2023. "Apical Sealing and Bioactivity of an Experimental Gutta-Percha Containing Niobium Phosphate Bioglass" Polymers 15, no. 7: 1679. https://doi.org/10.3390/polym15071679