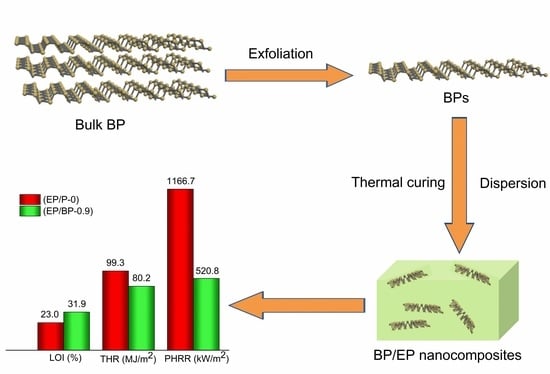
Flame Retardancy of Epoxy Resins Modified with Few-Layer Black Phosphorus
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Few-Layer BPs
2.3. Preparation of BP-Modified Epoxy Resins
2.4. Characterization
3. Results and Discussion
3.1. Characterization of BPs
3.2. Dispersibility of BPs and RP in Epoxy Composites
3.3. Flame-Retardant Properties
3.4. Thermal Properties
3.5. Mode of Action for Flame Retardancy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Credico, B.D.; Levi, M.; Turri, S. An efficient method for the output of new self-repairing materials through a reactive isocyanate encapsulation. Eur. Polym. J. 2013, 49, 2467–2476. [Google Scholar] [CrossRef]
- Ahmadi, Z. Nanostructured epoxy adhesives: A review. Prog. Org. Coat. 2019, 135, 449–453. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Khagokpam, G.K.S.; Halder, S. Paraffin wax microsphere embedded epoxy composites for potential thermal management in electronic devices. High Perform. Polym. 2018, 31, 767–777. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent Developments in Different Types of Flame Retardants and Effect on Fire Retardancy of Epoxy Composite. Polym.-Plast. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms-Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [Green Version]
- Mariappan, T.; Wilkie, C.A. Flame retardant epoxy resin for electrical and electronic applications. Fire Mater. 2014, 38, 588–598. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, M.; Qu, L.; Sun, G. Effect of phosphorus-containing flame retardants on flame retardancy and thermal stability of tetrafunctional epoxy resin. Polym. Adv. Technol. 2015, 26, 1531–1536. [Google Scholar] [CrossRef]
- Zarybnicka, L.; Bacovska, R.; Spacek, V.; Rychly, J.; Vecera, M.; Alberti, M. Preparation and Characterization of Cured Epoxy Resin with Hexachloro-Cyclo-Triphosphazene. Polym.-Plast. Technol. Eng. 2016, 56, 153–160. [Google Scholar] [CrossRef]
- Wang, C.S.; Lin, C.H. Synthesis and Properties of Phosphorus-Containing Epoxy Resins by Novel Method. J. Polym. Sci. A Polym. Chem. 1999, 37, 3903–3909. [Google Scholar] [CrossRef]
- Chi, Z.; Guo, Z.; Xu, Z.; Zhang, M.; Li, M.; Shang, L.; Ao, Y. A DOPO-based phosphorus-nitrogen flame retardant bio-based epoxy resin from diphenolic acid: Synthesis, flame-retardant behavior and mechanism. Polym. Degrad. Stab. 2020, 176, 109151. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, D.-Y.; Jian, R.-K.; Liu, Z.; Huang, G. Chemical structure construction of DOPO-containing compounds for flame retardancy of epoxy resin: A review. Prog. Org. Coat. 2023, 175, 107316. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Rosu, L.; Bifulco, A.; Rosu, D.; Mustata, F.; Gaan, S. Recent advances in flame retardant epoxy systems from reactive DOPO–based phosphorus additives. Polym. Degrad. Stab. 2022, 202, 110020. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Balabanovich, A.I.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.M.; Sandler, J.K.W.; Altstädt, V. Pyrolysis and fire behaviour of epoxy systems containing a novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-(DOPO)-based diamino hardener. Eur. Polym. J. 2008, 44, 704–715. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Huang, S.; Tian, X.; Song, L.; Yu, Q.; Wang, Z. Performance comparison of flame retardant epoxy resins modified by DPO–PHE and DOPO–PHE. Polym. Degrad. Stab. 2018, 156, 89–99. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Synthesis, characterization, and cure properties of phosphorus-containing epoxy resins for flame retardance. Eur. Polym. J. 2004, 40, 385–395. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, L.; Qiu, S.; Zhou, X.; Gui, Z.; Hu, Y. DOPO-Modified Two-Dimensional Co-Based Metal-Organic Framework: Preparation and Application for Enhancing Fire Safety of Poly(lactic acid). ACS Appl. Mater. Interfaces 2018, 10, 8274–8286. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, X.; Wu, J.; Wei, M.; Yu, Q.; Xiujuan, T.; Wang, Z. Performance comparison of epoxy resins modified with diphenylphosphine oxide and DOPO. Fire Mater. 2019, 43, 892–902. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, J.; Liu, Z.; Gu, Y.; Luan, G.; Sun, H.; Yu, Q.; Zhang, S.; Wang, Z. Effect of ethyl-bridged diphenylphosphine oxide on flame retardancy and thermal properties of epoxy resin. Polym. Adv. Technol. 2020, 31, 1426–1436. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, H.; Chen, J.; Wang, X.; Chen, L.; Ran, D.; Wang, Z.; Zeng, P. A new phosphorus flame-retard curing agent for epoxy resin/anhydride system. Polym. Adv. Technol. 2021, 33, 927–936. [Google Scholar] [CrossRef]
- Schäfer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Döring, M. Synthesis and properties of flame-retardant epoxy resins based on DOPO and one of its analog DPPO. J. Appl. Polym. Sci. 2007, 105, 685–696. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Song, T.; Jiao, Q.; Yang, R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stab. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. A Novel Multifunctional Organic-Inorganic Hybrid Curing Agent with High Flame-Retardant Efficiency for Epoxy Resin. ACS Appl. Mater. Interfaces 2015, 7, 17919–17928. [Google Scholar] [CrossRef]
- Dogan, M.; Murat Unlu, S. Flame retardant effect of boron compounds on red phosphorus containing epoxy resins. Polym. Degrad. Stab. 2014, 99, 12–17. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Deng, Y.; Ye, P.D. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Brent, J.R.; Savjani, N.; Lewis, E.A.; Haigh, S.J.; Lewis, D.J.; O’Brien, P. Production of few-layer phosphorene by liquid exfoliation of black phosphorus. Chem. Commun. 2014, 50, 13338–13341. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Sun, H.; Miao, L.; Chen, X.; Han, M.; Sun, J.; Liu, S.; Li, L.; Cheng, F.; Chen, J. Facile preparation of NH2-functionalized black phosphorene for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 2494–2499. [Google Scholar] [CrossRef]
- Erande, M.B.; Pawar, M.S.; Late, D.J. Humidity Sensing and Photodetection Behavior of Electrochemically Exfoliated Atomically Thin-Layered Black Phosphorus Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef]
- Ren, X.; Lian, P.; Xie, D.; Yang, Y.; Mei, Y.; Huang, X.; Wang, Z.; Yin, X. Properties, preparation and application of black phosphorus/phosphorene for energy storage: A review. J. Mater. Sci. 2017, 52, 10364–10386. [Google Scholar] [CrossRef]
- Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Yang, Y.; Wang, Y.; Wang, Z. A Novel Application of Phosphorene as a Flame Retardant. Polymers 2018, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Cai, T.; He, L.; Chu, F.; Mu, X.; Han, L.; Hu, Y.; Wang, B.; Hu, W. Natural antioxidant functionalization for fabricating ambient-stable black phosphorus nanosheets toward enhancing flame retardancy and toxic gases suppression of polyurethane. J. Hazard. Mater. 2020, 387, 121971. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zou, B.; Sheng, H.; Guo, W.; Wang, J.; Zhao, Y.; Wang, W.; Yuen, R.K.K.; Kan, Y.; Hu, Y. Electrochemically Exfoliated Functionalized Black Phosphorene and Its Polyurethane Acrylate Nanocomposites: Synthesis and Applications. ACS Appl. Mater. Interfaces 2019, 11, 13652–13664. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Flame-retardant and solid-solid phase change composites based on dopamine-decorated BP nanosheets/Polyurethane for efficient solar-to-thermal energy storage. Renew. Energy 2021, 164, 1–10. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.K.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface functionalization of few-layer black phosphorene and its flame retardancy in epoxy resin. Chem. Eng. J. 2020, 382, 122991. [Google Scholar] [CrossRef]
- Zou, B.; Qiu, S.; Ren, X.; Zhou, Y.; Zhou, F.; Xu, Z.; Zhao, Z.; Song, L.; Hu, Y.; Gong, X. Combination of black phosphorus nanosheets and MCNTs via phosphoruscarbon bonds for reducing the flammability of air stable epoxy resin nanocomposites. J. Hazard. Mater. 2020, 383, 121069. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, J.; Qian, L.; Li, J. Effect of gas-condensed phase synergistic system of 9,10-dihydro-9-oxo-10-phosphaphenanthrene-10-oxide and polydopamine on flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2020, 138, 49698. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, X.; Tang, Y.; Liu, Y.; Wei, J.; Chen, S.; Leow, W.R.; Li, W.; Liu, Y.; Deng, J.; et al. Wet-Chemical Processing of Phosphorus Composite Nanosheets for High-Rate and High-Capacity Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502409. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2600–2604. [Google Scholar] [CrossRef]
- Bagheri, S.; Mansouri, N.; Aghaie, E. Phosphorene: A new competitor for graphene. Int. J. Hydrogen Energy 2016, 41, 4085–4095. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Wachtendorf, V.; Klack, P.; Qian, L.; Liu, Z.; Schartel, B. Improved flame retardancy by synergy between cyclotetrasiloxane and phosphaphenanthrene/triazine compounds in epoxy thermoset. Polym. Int. 2017, 66, 1883–1890. [Google Scholar] [CrossRef]
- Wu, W.; Xu, Y.; Wu, H.; Chen, J.; Li, M.; Chen, T.; Hong, J.; Dai, L. Synthesis of modified graphene oxide and its improvement on flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 47710–47720. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Wu, J.; Song, L.; Han, Y.; Wang, Z.; Yu, Q. Glass fiber reinforced PET modified by few-layer black phosphorus. Polym. Adv. Technol. 2021, 32, 3515–3522. [Google Scholar] [CrossRef]
- Garth, K.; Klinkowski, C.; Fuhr, O.; Döring, M. Synthesis of a new phosphorylated ethylamine, thereon based phosphonamidates and their application as flame retardants. Heteroat. Chem. 2017, 28, e21407. [Google Scholar] [CrossRef]
- Sang, B.; Li, Z.-w.; Li, X.-h.; Yu, L.-g.; Zhang, Z.-j. Graphene-based flame retardants: A review. J. Mater. Sci. 2016, 51, 8271–8295. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Yu, B.; Wang, B.; Yuan, B.; Shi, Y.; Hu, Y.; Yuen, R.K.K. Novel organic–inorganic flame retardants containing exfoliated graphene: Preparation and their performance on the flame retardancy of epoxy resins. J. Mater. Chem. A 2013, 1, 6822–6830. [Google Scholar] [CrossRef]
- Wang, R.; Zhuo, D.; Weng, Z.; Wu, L.; Cheng, X.; Zhou, Y.; Wang, J.; Xuan, B. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J. Mater. Chem. A 2015, 3, 9826–9836. [Google Scholar] [CrossRef]
- Täuber, K.; Marsico, F.; Wurm, F.R.; Schartel, B. Hyperbranched poly(phosphoester)s as flame retardants for technical and high performance polymers. Polym. Chem. 2014, 5, 7042–7053. [Google Scholar] [CrossRef]










| Sample | tPHRR (s) | PHRR (kW/m2) | THR (MJ/m2) | av-EHC (MJ/kg) | av-CO (kg/kg) | av-CO2 (kg/kg) | TML (wt.%) | TTI (s) | Char Residual (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| EP/P-0 | 115 | 1166.7 | 99.3 | 23.3 | 0.09 | 1.68 | 96.4 | 40 | 3.55 |
| EP/BP-0.9 | 130 | 520.8 | 80.2 | 19.0 | 0.12 | 1.24 | 86.0 | 38 | 13.20 |
| EP/RP-0.9 | 105 | 673.9 | 88.2 | 23.7 | 0.10 | 1.51 | 89.8 | 38 | 10.19 |
| FRs | BP-Bulk | BP-PZN | BP | BP@MF | BPs |
|---|---|---|---|---|---|
| Fraction (wt.%) | 2 | 2 | 1.2 | 1.2 | 0.9 |
| φLOI | - | - | 5.1% | 21.1% | 43.0% |
| φPHRR | 24.4% | 29.7% | 32.8% | 36.1% | 61.5% |
| φTHR | 21.8% | 31.8% | 2.3% | 10.5% | 21.4% |
| UL-94 | V-0 | - | NR | V-0 | V-0 |
| Matrix | E-44 | E-44 | E-51 | E-51 | E-44 |
| Ref. | [34] | [31] | This work | ||
| Sample | Flame Inhibition Effect | Charring Effect | Barrier and Protective Effect |
|---|---|---|---|
| EP/BP-0.9 | 18.45% | 10.79% | 44.73% |
| Sample | T5% (°C) | Char Yeild at 700 °C (wt.%) | Tg (°C) | Storage Modulus (MPa) | νe (×103 mol/m3) | |
|---|---|---|---|---|---|---|
| 50 °C | Tg + 40 °C | |||||
| EP/P-0 | 377.5 | 13.4 | 189 | 1331.87 | 10.36 | 1.59 |
| EP/BP-0.3 | 367.2 | 18.2 | 186 | 1388.82 | 11.18 | 1.68 |
| EP/BP-0.6 | 359.9 | 19.8 | 190 | 1141.44 | 9.48 | 1.42 |
| EP/BP-0.9 | 357.6 | 21.1 | 197 | 1470.61 | 9.18 | 1.38 |
| EP/RP-0.9 | 374.6 | 16.0 | 188 | 1579.31 | 12.80 | 1.92 |
| EP/RP-15 | 359.9 | 26.2 | 191 | 1449.56 | 13.94 | 2.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, Y.; Li, J.; Xiao, Y.; Mu, W.; Wang, Z.; Song, L.; Yu, J. Flame Retardancy of Epoxy Resins Modified with Few-Layer Black Phosphorus. Polymers 2023, 15, 1655. https://doi.org/10.3390/polym15071655
Zhao Y, Li Y, Li J, Xiao Y, Mu W, Wang Z, Song L, Yu J. Flame Retardancy of Epoxy Resins Modified with Few-Layer Black Phosphorus. Polymers. 2023; 15(7):1655. https://doi.org/10.3390/polym15071655
Chicago/Turabian StyleZhao, Yongzheng, Yan Li, Jiaxuan Li, Yifan Xiao, Wenmin Mu, Zhongwei Wang, Liang Song, and Jinhong Yu. 2023. "Flame Retardancy of Epoxy Resins Modified with Few-Layer Black Phosphorus" Polymers 15, no. 7: 1655. https://doi.org/10.3390/polym15071655