Nanotopography of Polystyrene/Poly(methyl methacrylate) for the Promotion of Patient Specific Von Willebrand Factor Entrapment and Platelet Adhesion in a Whole Blood Microfluidic Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
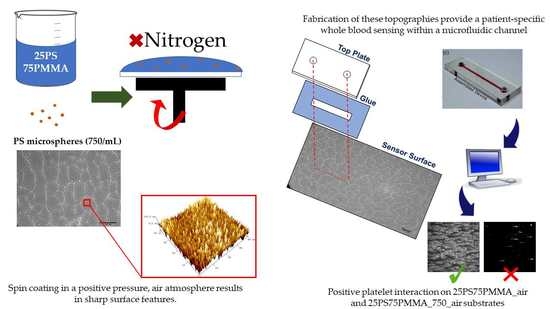
2.2. Spin Coating
2.3. Surface Characterisation
2.4. Haematological Assessment
2.5. Dynamic Platelet Function Assay
2.6. Dynamic Platelet Analysis Algorithm
3. Results
3.1. Characterisation of Air-Spun PS/PMMA and PS/PMMA_750 Thin Films
3.2. Haematological Assessment
3.3. Dynamic Analysis of Platelet Behaviours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Favaloro, E.J.; Pasalic, L.; Curnow, J. Laboratory Tests Used to Help Diagnose von Willebrand Disease: An Update. Pathology 2016, 48, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Castaman, G.; Dini, E. Epidemiological Investigation of the Prevalence of von Willebrands Disease. Blood 1987, 69, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bykowska, K.; Ceglarek, B. Clinical Significance of Slightly Reduced von Willebrand Factor Activity (30–50 IU/DL). Pol. Arch. Intern. Med. 2020, 130, 225–231. [Google Scholar] [CrossRef]
- Ng, C.; Motto, D.G.; Di Paola, J. Diagnostic Approach to von Willebrand Disease. Blood 2015, 125, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis, T. The Diagnosis, Treatment and Management of von Willebrand Disease; U.S. Department of Health & Human Services: Washington, DC, USA, 2007; pp. 1–111.
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D. ISTH/SSC Bleeding Assessment Tool: A Standardized Questionnaire and a Proposal for a New Bleeding Score for Inherited Bleeding Disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Sadler, J.E.; Budde, U.; Eikenboom, J.C.J.; Favaloro, E.J.; Hill, F.G.H.; Holmberg, L.; Ingerslev, J.; Lee, C.A.; Lillicrap, D.; Mannucci, P.M. Update on the Pathophysiology and Classification of von Willebrand Disease: A Report of the Subcommittee on von Willebrand Factor. J. Thromb. Haemost. 2006, 4, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Bharati, K.P.; Prashanth, U.R. Von Willebrand Disease: An Overview. Indian J. Pharm. Sci. 2011, 73, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.C.; Flood, V.H. Laboratory Diagnosis of von Willebrand Disease. Int. J. Lab. Hematol. 2015, 37, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Hamidian, K.; Barani, M.; Adeli-Sardou, M.; Sarani, M.; Daliran, S.; Oveisi, A.R. Evaluation of Cytotoxicity, Loading, and Release Activity of Paclitaxel Loaded-Porphyrin Based Metal-Organic Framework (PCN-600). Heliyon 2023, 9, e12634. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Xiao, M.; Wang, D.; Qu, Y.; Zou, L.; Zheng, C.; Zhang, J. Oral Colon-Targeted Mucoadhesive Micelles with Enzyme-Responsive Controlled Release of Curcumin for Ulcerative Colitis Therapy. Chin. Chem. Lett. 2022, 33, 4924–4929. [Google Scholar] [CrossRef]
- Khosravi Ardakani, H.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent Progress in Nanobiosensors for Precise Detection of Blood Glucose Level. Biochem. Res. Int. 2022, 2022, 2964705. [Google Scholar] [CrossRef]
- James, P.D.; Lillicrap, D. Von Willebrand Disease: Clinical and Laboratory Lessons Learned from the Large von Willebrand Disease Studies. Am. J. Hematol. 2012, 87, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P. Progress in the Assessment of Platelet Function. Br. J. Haematol. 2000, 111, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. The Growing Complexity of Platelet Aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef] [Green Version]
- Roest, M.; Reininger, A.; Zwaginga, J.J.; King, M.R.; Heemskerk, J.W.M. Flow Chamber-Based Assays to Measure Thrombus Formation in Vitro: Requirements for Standardization. J. Thromb. Haemost. 2011, 9, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- Lucitt, M.B.; O’Brien, S.; Cowman, J.; Meade, G.; Basabe-Desmonts, L.; Somers, M.; Kent, N.; Ricco, A.J.; Kenny, D. Assaying the Efficacy of Dual-Antiplatelet Therapy: Use of a Controlled-Shear-Rate Microfluidic Device with a Well-Defined Collagen Surface to Track Dynamic Platelet Adhesion. Anal. Bioanal. Chem. 2013, 405, 4823–4834. [Google Scholar] [CrossRef]
- Hansen, R.R.; Wufsus, A.R.; Barton, S.T.; Onasoga, A.A.; Johnson-Paben, R.M.; Neeves, K.B. High Content Evaluation of Shear Dependent Platelet Function in a Microfluidic Flow Assay. Ann. Biomed. Eng. 2013, 41, 250–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowman, J.J. The Dynamic Platelet Function Assay (DPFA): Assessment of Platelet Translocation Behaviour on von Willebrand Factor (VWF) in Pregnancy, Neonates and Adults. Doctoral Dissertation, Royal Colleage of Surgeons in Ireland, Dublin, Ireland, 2015. [Google Scholar]
- Cowman, J.; Müllers, S.; Dunne, E.; Ralph, A.; Ricco, A.J.; Malone, F.D.; Kenny, D. Platelet Behaviour on von Willebrand Factor Changes in Pregnancy: Consequences of Haemodilution and Intrinsic Changes in Platelet Function. Sci. Rep. 2017, 7, 6354. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.; Kenny, D. Why Is Blood Group a Risk Marker for Myocardial Infarction? Doctoral Dissertation, Royal College of Surgeons in Ireland, Dublin, Ireland, 2016. [Google Scholar]
- Basabe-Desmonts, L.; Meade, G.; Kenny, D. New Trends in Bioanalytical Microdevices to Assess Platelet Function. Expert Rev. Mol. Diagn. 2010, 10, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Kent, N.J.; Basabe-Desmonts, L.; Meade, G.; MacCraith, B.D.; Corcoran, B.G.; Kenny, D.; Ricco, A.J. Microfluidic Device to Study Arterial Shear-Mediated Platelet-Surface Interactions in Whole Blood: Reduced Sample Volumes and Well-Characterised Protein Surfaces. Biomed. Microdevices 2010, 12, 987–1000. [Google Scholar] [CrossRef]
- Qi, Q.M.; Dunne, E.; Oglesby, I.; Schoen, I.; Ricco, A.J.; Kenny, D.; Shaqfeh, E.S.G. In Vitro Measurement and Modeling of Platelet Adhesion on VWF-Coated Surfaces in Channel Flow. Biophys. J. 2019, 116, 1136–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, A.; Somers, M.; Cowman, J.; Voisin, B.; Hogan, E.; Dunne, H.; Dunne, E.; Byrne, B.; Kent, N.; Ricco, A.J.; et al. Computational Tracking of Shear-Mediated Platelet Interactions with von Willebrand Factor. Cardiovasc. Eng. Technol. 2016, 7, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, B.; Ricco, A.J.; Kent, N.J.; Basabe-Desmonts, L.; Lee, L.P.; MacCraith, B.D.; Kenny, D.; Meade, G. Integrated System Investigating Shear-Mediated Platelet Interactions with von Willebrand Factor Using Microliters of Whole Blood. Anal. Biochem. 2010, 405, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Cowman, J.; Quinn, N.; Geoghegan, S.; Müllers, S.; Oglesby, I.; Byrne, B.; Somers, M.; Ralph, A.; Voisin, B.; Ricco, A.J.; et al. Dynamic Platelet Function on von Willebrand Factor Is Different in Preterm Neonates and Full-Term Neonates: Changes in Neonatal Platelet Function. J. Thromb. Haemost. 2016, 14, 2027–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, D.; Meenan, B.J. Bespoke Nanotopography for the Selective Capture of VWF from Whole Blood at Arterial Shear: A Potential Diagnostic Platflorm. Doctoral Dissertation, Ulster University, Coleraine, UK, 2016. [Google Scholar]
- Ward, J.; Dunne, E.; Bishop, D.; Boyd, A.; Kenny, D.; Meenan, B.J. Entrapment of Autologous von Willebrand Factor on Polystyrene/Poly(Methyl Methacrylate) Demixed Surfaces. Polymers 2017, 9, 700. [Google Scholar] [CrossRef] [Green Version]
- Minelli, C.; Kikuta, A.; Tsud, N.; Ball, M.D.; Yamamoto, A. A Micro-Fluidic Study of Whole Blood Behaviour on PMMA Topographical Nanostructures. J. Nanobiotechnol. 2008, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Association, W.M. World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef] [Green Version]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1992; ISBN 0471 935921. [Google Scholar]
- Kettle, J.; Ding, Z.; Horie, M.; Smith, G.C. XPS Analysis of the Chemical Degradation of PTB7 Polymers for Organic Photovoltaics. Org. Electron. 2016, 39, 222–228. [Google Scholar] [CrossRef]
- Strawhecker, K.E.; Kumar, S.K.; Douglas, J.F.; Karim, A. The Critical Role of Solvent Evaporation on the Roughness of Spin-Cast Polymer Films. Macromolecules 2001, 34, 4669–4672. [Google Scholar] [CrossRef]
- Chapman, N.; Chapman, M.; Euler, W.B. Modeling of Poly(Methylmethacrylate) Viscous Thin Films by Spin-Coating. Coatings 2021, 11, 198. [Google Scholar] [CrossRef]
- Fleming, G.; Aveyard, J.; Fothergill, J.L.; McBride, F.; Raval, R.; D’Sa, R.A. Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation. Polymers 2019, 11, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Han, Y.; An, L. Surface Morphology Control of Immiscible Polymer-Blend Thin Films. Polymer 2003, 44, 8155–8165. [Google Scholar] [CrossRef]
- Zuyderhoff, E.M.; Dekeyser, C.M.; Rouxhet, P.G.; Dupont-Gillain, C.C. An AFM, XPS and Wettability Study of the Surface Heterogeneity of PS/PMMA-r-PMAA Demixed Thin Films. J. Colloid Interface Sci. 2008, 319, 63–71. [Google Scholar] [CrossRef] [PubMed]




| Name | Donor 1 | Haematocrit (HCT) | 38.9% |
| Age | 24 | Platelet Count (PLT) | 260 × 103/µL |
| Blood Type | O | Mean Platelet Volume (MPV) | 9.4 fL |
| Gender | Female | Platelet-Large Cell Ratio (P-LCR) | 17.6% |
| Parameter | Biological Relevance |
|---|---|
| Platelet adhesion rate (×103/s) | Rate of change in platelet adhesion to the surface, which is calculated by plotting a regression line against the cumulative number of platelets. A higher platelet adhesion rate indicates that platelets are ‘stickier’ or more likely to form a thrombus. |
| Mean translocating velocity (µm/s) | Mean velocity is an indication of how fast platelets are translocating. A reduction in platelet translocation speed may indicate increased signalling within the platelet, causing faster activation of GP IIb/IIIa, which slows down platelets and leads to stable adhesion. |
| Mean translocating distance (µm) | Average distance travelled by translocating platelets. A decrease in this value can indicate increased signalling within the platelet, which leads to faster activation of GP IIb/IIIa, reducing the distance a platelet travels before stably adhering to the surface. |
| Fraction of stably adhered platelets | Total number of platelets that are stably adhered to the surface as a fraction of all detected platelet tracks. This is defined as platelets moving less than 1.5× their own radius over the whole duration of the movie. In biological terms, this refers to the number of platelets that initially interact with autologous vWF and remain adhered to the surface. |
| Substrate | C 1s | O 1s | ||||
|---|---|---|---|---|---|---|
| C-H | C-C | C-O | C=O | O=C | O-C | |
| 25PS75 PMMA | 33.48 ± 1.17 | 20.97 ± 0.67 | 28.27 ± 1.62 | 17.28 ± 0.37 | 45.22 ± 0.68 | 54.78 ± 0.68 |
| 25PS75 PMMA_ 750 | 34.24 ± 3.42 | 22.35 ± 0.77 | 25.53 ± 3.04 | 17.88 ± 0.47 | 44.20 ± 0.70 | 55.80 ± 0.70 |
| Substrate | Sample | Surface Coverage (%) | Average (%) ± SD |
|---|---|---|---|
| 25PS75PMMA | 1 | 8.00 | 17.30 ± 6.80 |
| 2 | 14.10 | ||
| 3 | 20.00 | ||
| 4 | 18.00 | ||
| 5 | 26.20 | ||
| 25PS75PMMA_750 | 1 | 27.70 | 40.20 ± 13.30 |
| 2 | 26.90 | ||
| 3 | 48.40 | ||
| 4 | 39.80 | ||
| 5 | 57.90 |
| Dynamic Parameters | 25PS75PMMA | 25PS75PMMA_750 | p-Value |
|---|---|---|---|
| Total number of tracks per FOV | 168 ± 44 | 474 ± 331 | 0.07 |
| Fraction of stably adhered platelets | 0.75 ± 0.03 | 0.78 ± 0.05 | 0.24 |
| Mean translocating distance (μm) | 2.22 ± 0.31 | 2.13 ± 0.38 | 0.68 |
| Mean translocating velocity (μm/s) | 1.44 ± 1.06 | 1.21 ± 0.64 | 0.69 |
| Platelet adhesion rate (×103 s−1) | 0.69 ± 0.15 | 1.65 ± 1.27 | 0.14 |
| Platelet detachment rate (s−1) | 1.30 ± 1.89 | 2.20 ± 1.33 | 0.41 |
| Fraction platelets sticking | 0.47 ± 0.18 | 0.49 ± 0.09 | 0.79 |
| Platelet Indices * | |||
| Platelet count (×103/μL) | 224 | 260 | |
| Haematocrit (%) | 31.2 | 38.9 | |
| vWF (IU/mL) | 47.6 | 47.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, J.; Dunne, E.; Schoen, I.; Boyd, A.R.; Kenny, D.; Meenan, B.J. Nanotopography of Polystyrene/Poly(methyl methacrylate) for the Promotion of Patient Specific Von Willebrand Factor Entrapment and Platelet Adhesion in a Whole Blood Microfluidic Assay. Polymers 2023, 15, 1580. https://doi.org/10.3390/polym15061580
Ward J, Dunne E, Schoen I, Boyd AR, Kenny D, Meenan BJ. Nanotopography of Polystyrene/Poly(methyl methacrylate) for the Promotion of Patient Specific Von Willebrand Factor Entrapment and Platelet Adhesion in a Whole Blood Microfluidic Assay. Polymers. 2023; 15(6):1580. https://doi.org/10.3390/polym15061580
Chicago/Turabian StyleWard, Joanna, Eimear Dunne, Ingmar Schoen, Adrian R. Boyd, Dermot Kenny, and Brian J. Meenan. 2023. "Nanotopography of Polystyrene/Poly(methyl methacrylate) for the Promotion of Patient Specific Von Willebrand Factor Entrapment and Platelet Adhesion in a Whole Blood Microfluidic Assay" Polymers 15, no. 6: 1580. https://doi.org/10.3390/polym15061580