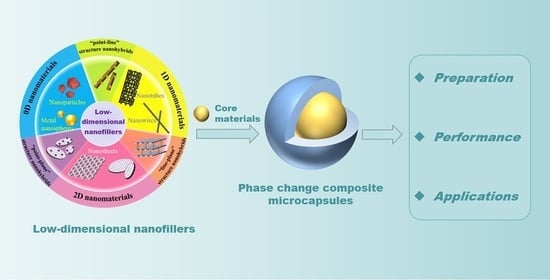
Phase Change Composite Microcapsules with Low-Dimensional Thermally Conductive Nanofillers: Preparation, Performance, and Applications
Abstract
:1. Introduction
2. Composition and Preparation of MEPCMs
2.1. Core Materials of MEPCMs
2.2. Shell Materials of MEPCMs
2.2.1. Organic Shells
2.2.2. Inorganic Shells
2.2.3. Organic-Inorganic Hybrid Shells
2.3. Preparation Strategies of MEPCMs
2.3.1. Physical Method
2.3.2. Chemical Method
2.3.3. Physical-Chemical Method
3. Thermal Conductivity Enhancement of PCMs with Low-Dimensional Nanofillers
3.1. Zero-Dimensional Nanofillers
3.2. One-Dimensional Nanofillers
3.3. Two-Dimensional Nanofillers
3.4. Low-Dimensional Hybrid Nanofillers
4. Thermal Management Applications
4.1. Electronic Devices
4.2. Heat-Adjusting Fiber
4.3. Energy-Saving Buildings
4.4. Others
5. Conclusions and Future Perspectives
- (1)
- The incorporation of low-dimensional thermally conductive nanofillers in MEPCMs can indeed effectively enhance the thermal conductivity of PCMs, which is critically significant to promote their thermal charging and discharging in practical thermal management systems. However, a quantitative theory between low-dimensional nanofillers and thermal conductivity is still lacking. Moreover, it is also challenging to achieve the uniform dispersion of nanofillers in MEPCMs, and the resultant high interfacial thermal resistance causes the superiority of low-dimensional nanofillers have not been fully exploited. In addition, the energy storage density of MEPCMs is usually lower than that of pristine PCMs in many cases. It is challenging to achieve a promising balance between high energy storage density, low leakage, superior thermal conductivity, and highly efficient energy conversion. It is highly advisable to explore some novel strategy to optimize the interfaces and aligned heat transfer networks.
- (2)
- Although the MEPCMs reinforced with low-dimensional nanofillers exhibit many impressive properties, their large-scale production is still challenging due to the complicated manufacturing process and high costs. For example, BNNS, with high in-plane thermal conductivity, distinctive chemical inertness, and excellent inherent electrical insulation, is very suitable for developing advanced MEPCMs, which exhibits promising applications in electronic devices. However, it is difficult and time-cost to obtain high-quality and defect-free BN nanosheets with large lateral sizes. Besides, as for the encapsulation strategies, simple and feasible synthetic methods are worthy exploring by high-throughput simulation and machine learning. Moreover, with the increasing environmental awareness, the eco-friendly raw materials and solvent-free synthesis also become crucial.
- (3)
- Versatile MEPCMs for multi-purpose thermal management applications should be investigated. Until now, most research focus of MEPCMs containing low-dimensional nanofillers was placed on achieving synergetic enhancement of higher thermal conductivity, efficient energy conversion, and larger energy storage density, while their cycle stability, supercooling, and phase separation problems are usually ignored. Besides, the other functions of MEPCMs such as humidity control, flame resistance, electromagnetic shielding, flexible sensing should also receive adequate attentions for satisfying the specific requirements of their widespread applications in the future. Moreover, a database on MEPCMs with low-dimensional nanofillers should be established for better design of MEPCMs-based thermal management systems with targeted thermal performance even though there is still a long way to achieve this goal. In summary, by systematically summarizing the relevant achievements and exploring their preparation–structure–properties-applications relationships, we hope this review provide in-depth insights and meaningful enlightenment for the architectural design and multifunction of advanced MEPCMs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Waterson, M. The characteristics of electricity storage, renewables and markets. Energy Policy 2017, 104, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Fernández, A.; Martínez, M.; Segarra, M.; Martorell, I.; Cabeza, L. Selection of materials with potential in sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2010, 94, 1723–1729. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Xie, B.-H.; Yang, M.-B.; Yang, W. High-performance composite phase change materials for energy conversion based on macroscopically three-dimensional structural materials. Mater. Horiz. 2019, 6, 250–273. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Du, Y.; Lu, X.; Qu, J. Shape-stable composite phase change materials encapsulated by bio-based balsa wood for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111187. [Google Scholar] [CrossRef]
- Li, X.; Sheng, M.; Gong, S.; Wu, H.; Chen, X.; Lu, X.; Qu, J. Flexible and multifunctional phase change composites featuring high-efficiency electromagnetic interference shielding and thermal management for use in electronic devices. Chem. Eng. J. 2022, 430, 132928. [Google Scholar] [CrossRef]
- Huang, J.; Wu, B.; Lyu, S.; Li, T.; Han, H.; Li, D.; Wang, J.-K.; Zhang, J.; Lu, X.; Sun, D. Improving the thermal energy storage capability of diatom-based biomass/polyethylene glycol composites phase change materials by artificial culture methods. Sol. Energy Mater. Sol. Cells 2021, 219, 110797. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, C.; Wu, H.; Li, X.; Lu, X.; Qu, J. Manufacturing. Large-scale preparation of flexible phase change composites with synergistically enhanced thermally conductive network for efficient low-grade thermal energy recovery and utilization. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106770. [Google Scholar] [CrossRef]
- Yang, A.-S.; Cai, T.; Su, L.; Li, Y.; He, F.; Zhang, Q.-P.; Zhou, Y.; He, R.; Zhang, K.; Yang, W. Review on Organic Phase Change Materials for Sustainable Energy Storage. Sustain. Energy Fuels 2022, 6, 5045–5071. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, X.-Y.; Han, S.; Zhang, X.-L.; Guo, Y.-D.; Wang, Y.-Y.; Tan, Y.-Q.; Han, N.-X.; Li, W. Preparation and physicochemical properties of microcapsules containing phase-change material with graphene/organic hybrid structure shells. J. Mater. Chem. A 2017, 5, 23937–23951. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Ji, R.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Huang, P.; Li, B.; Sun, L. Design and synthesis of novel microencapsulated phase change materials with enhancement of thermal conductivity and thermal stability: Self-assembled boron nitride into shell materials. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124225. [Google Scholar] [CrossRef]
- Huang, H.; Shi, T.; He, R.; Wang, J.; Chu, P.K.; Yu, X.F. Phase-Changing Microcapsules Incorporated with Black Phosphorus for Efficient Solar Energy Storage. Adv. Sci. 2020, 7, 2000602. [Google Scholar] [CrossRef] [PubMed]
- Altun-Anayurt, R.; Alay-Aksoy, S.; Alkan, C.; Demirbag, S.; Tözüm, M.S. Development of thermo-regulating fabrics using PCM microcapsules with poly (methyl methacrylate-co-2-hydroxy ethyl methacrylate) shell and n-alkane core. Int. J. Cloth. Sci. Technol. 2019, 31, 65–79. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.-M.; Gschwander, S.; Haussmann, T. Micro-encapsulated phase-change materials integrated into construction materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, H.; Zhang, L.; Xu, B.; Xiao, F. Fabrication of novel slurry containing graphene oxide-modified microencapsulated phase change material for direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2018, 188, 73–80. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Wang, J.; Tang, G.; Song, G. Fabrication, thermal properties and thermal stabilities of microencapsulated n-alkane with poly (lauryl methacrylate) as shell. Thermochim. Acta 2015, 620, 10–17. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Rodriguez-Ubinas, E.; Ruiz-Valero, L.; Vega, S.; Neila, J. Applications of phase change material in highly energy-efficient houses. Energy Build. 2012, 50, 49–62. [Google Scholar] [CrossRef]
- Huang, L.; Ning, J.; Yang, Y.; Tian, C.; Lv, J.; Zeng, F.; Liu, Q.; Zhao, F.; Cai, X.; Kong, W. Thermal-Conductive, Dynamic Cross-Linked Solid–Solid Phase Change Composites toward Sustainable Energy Utilization. Ind. Eng. Chem. Res. 2022, 61, 6448–6457. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar-thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Gou, J.; Liu, W. Feasibility study on a novel 3D vapor chamber used for Li-ion battery thermal management system of electric vehicle. Appl. Therm. Eng. 2019, 152, 362–369. [Google Scholar] [CrossRef]
- Farzanehnia, A.; Khatibi, M.; Sardarabadi, M.; Passandideh-Fard, M. Experimental investigation of multiwall carbon nanotube/paraffin based heat sink for electronic device thermal management. Energy Convers. Manag. 2019, 179, 314–325. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Luo, W.; Quan, X.; Li, H.; Huang, J.; Feng, W. High-energy and light-actuated phase change composite for solar energy storage and heat release. Surf. Interfaces 2021, 24, 101071. [Google Scholar] [CrossRef]
- Arıcı, M.; Bilgin, F.; Nižetić, S.; Karabay, H. PCM integrated to external building walls: An optimization study on maximum activation of latent heat. Appl. Therm. Eng. 2020, 165, 114560. [Google Scholar] [CrossRef]
- Hu, H. Recent advances of polymeric phase change composites for flexible electronics and thermal energy storage system. Compos. Part B Eng. 2020, 195, 108094. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, E.; Li, G. Study on transition characteristics of PEG/CDA solid–solid phase change materials. Polymer 2002, 43, 117–122. [Google Scholar] [CrossRef]
- Chandra, D.; Chellappa, R.; Chien, W.-M. Thermodynamic assessment of binary solid-state thermal storage materials. J. Phys. Chem. Solids 2005, 66, 235–240. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- He, B.; Martin, V.; Setterwall, F. Phase transition temperature ranges and storage density of paraffin wax phase change materials. Energy 2004, 29, 1785–1804. [Google Scholar] [CrossRef]
- Akgün, M.; Aydın, O.; Kaygusuz, K. Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Convers. Manag. 2007, 48, 669–678. [Google Scholar] [CrossRef]
- Hoshi, A.; Mills, D.R.; Bittar, A.; Saitoh, T.S. Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR. Sol. Energy 2005, 79, 332–339. [Google Scholar] [CrossRef]
- Zhou, D.; Eames, P. Thermal characterisation of binary sodium/lithium nitrate salts for latent heat storage at medium temperatures. Sol. Energy Mater. Sol. Cells 2016, 157, 1019–1025. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.; Sahin, A. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Ke, H. Phase diagrams, eutectic mass ratios and thermal energy storage properties of multiple fatty acid eutectics as novel solid-liquid phase change materials for storage and retrieval of thermal energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar]
- Zhu, K.; Qi, H.; Wang, S.; Zhou, J.; Zhao, Y.; Su, J.; Yuan, X. Preparation and characterization of melamine-formaldehyde resin micro-and nanocapsules filled with n-dodecane. J. Macromol. Sci. Part B 2012, 51, 1976–1990. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Q.; Li, Y.; He, F.; Zhou, Y.; He, R.; Fan, J.; Zhang, K.; Yang, W. Nanodiamond-Modified Microencapsulated Phase-Change Materials with Superhydrophobicity and High Light-to-Thermal Conversion Efficiency. Ind. Eng. Chem. Res. 2020, 59, 21736–21744. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, S.-B.; Zhang, Y.-Y.; Huang, Z. Physicochemical properties and mechanical characters of methanol-modified melamine-formaldehyde (MMF) shell microPCMs containing paraffin. Colloid Polym. Sci. 2011, 289, 111–119. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly (melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Huang, R.; Li, W.; Wang, J.; Zhang, X. Effects of oil-soluble etherified melamine-formaldehyde prepolymers on in situ microencapsulation and macroencapsulation of n-dodecanol. New J. Chem. 2017, 41, 9424–9437. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.-H.; Zeng, X.-R. Preparation, characterization, and thermal properties of microPCMs containing n-dodecanol by using different types of styrene-maleic anhydride as emulsifier. Colloid Polym. Sci. 2009, 287, 549–560. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chang, T.; Wang, J.; Wang, X.; Zhou, G. Phase change material microcapsules with melamine resin shell via cellulose nanocrystal stabilized Pickering emulsion in-situ polymerization. Chem. Eng. J. 2022, 428, 131164. [Google Scholar] [CrossRef]
- Mao, J.; Yang, H.; Zhou, X. In situ polymerization of uniform poly (urea–formaldehyde) microcapsules containing paraffins under the high-speed agitation without emulsifier. Polym. Bull. 2012, 69, 649–660. [Google Scholar] [CrossRef]
- Wang, G.; Xu, W.; Hou, Q.; Guo, S. Microwave-assisted synthesis of poly (urea-formaldehyde)/lauryl alcohol phase change energy storage microcapsules. Polym. Sci. Ser. B 2016, 58, 321–328. [Google Scholar] [CrossRef]
- Xin, C.; Tian, Y.; Wang, Y.; Huang, X. Effect of curing temperature on the performance of microencapsulated low melting point paraffin using urea-formaldehyde resin as a shell. Text. Res. J. 2014, 84, 831–839. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Lopez, V.; Cuenca, N.; Valverde, J.L. Poly (urea-formaldehyde) microcapsules containing commercial paraffin: In situ polymerization study. Colloid Polym. Sci. 2018, 296, 1449–1457. [Google Scholar] [CrossRef]
- Liu, C.; Ma, L.; Rao, Z.; Li, Y. Synthesis and thermal properties of magnesium sulfate heptahydrate/urea resin as thermal energy storage micro-encapsulated phase change material. J. Heat Transf. 2018, 140, 014501. [Google Scholar] [CrossRef]
- Huang, Z.-h.; Yu, X.; Li, W.; Liu, S.-x. Preparation of urea-formaldehyde paraffin microcapsules modified by carboxymethyl cellulose as a potential phase change material. J. For. Res. 2015, 26, 253–260. [Google Scholar] [CrossRef]
- Tohmura, S.-i.; Inoue, A.; Sahari, S.H. Influence of the melamine content in melamine-urea-formaldehyde resins on formaldehyde emission and cured resin structure. J. Wood Sci. 2001, 47, 451–457. [Google Scholar] [CrossRef]
- Rezvanpour, M.; Hasanzadeh, M.; Azizi, D.; Rezvanpour, A.; Alizadeh, M. Synthesis and characterization of micro-nanoencapsulated n-eicosane with PMMA shell as novel phase change materials for thermal energy storage. Mater. Chem. Phys. 2018, 215, 299–304. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.H.; Zeng, X.R.; Gao, X.N.; Zhang, Z.G. Poly(methyl methacrylate) copolymer nanocapsules containing phase-change material (n-dodecanol) prepared via miniemulsion polymerization. J. Appl. Polym. Sci. 2015, 132, 42334. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Biçer, A.; Altuntaş, A.; Bilgin, C. Micro/nanoencapsulated n-nonadecane with poly(methyl methacrylate) shell for thermal energy storage. Energy Convers. Manag. 2014, 86, 614–621. [Google Scholar] [CrossRef]
- Wang, T.Y.; Huang, J. Synthesis and characterization of microencapsulated sodium phosphate dodecahydrate. J. Appl. Polym. Sci. 2013, 130, 1516–1523. [Google Scholar] [CrossRef]
- Spernath, L.; Magdassi, S. Polyurea nanocapsules obtained from nano-emulsions prepared by the phase inversion temperature method. Polym. Adv. Technol. 2011, 22, 2469–2473. [Google Scholar] [CrossRef]
- Yang, J.M.; Kim, J.S. The microencapsulation of calcium chloride hexahydrate as a phase-change material by using the hybrid coupler of organoalkoxysilanes. J. Appl. Polym. Sci. 2018, 135, 45821. [Google Scholar] [CrossRef]
- Ghoghaei, M.S.; Mahmoudian, A.; Mohammadi, O.; Shafii, M.B.; Jafari Mosleh, H.; Zandieh, M.; Ahmadi, M.H. Calorimetry. A review on the applications of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2021, 145, 245–268. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Wang, Y.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Flexible and resilient photothermal polyurethane film from polydopamine-coated phase change microcapsules. Sol. Energy Mater. Sol. Cells 2021, 227, 111111. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Z.; Ma, W. Fabrication and characterization of a novel polyurethane microencapsulated phase change material for thermal energy storage. Prog. Org. Coat. 2021, 151, 106006. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Hu, D.; Wu, L. Synthesis and characterization of microencapsulated methyl laurate with polyurethane shell materials via interfacial polymerization in Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124958. [Google Scholar] [CrossRef]
- Yan, J.; Hu, D.; Ma, W.; Chen, W. Preparation and characterization of soy polyols-based PU shell microencapsulated phase change materials for reliable thermal energy storage. Int. J. Energy Res. 2022, 46, 23364–23376. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Döğüşcü, D.K.; Kızıl, Ç. Micro/nano encapsulated n-tetracosane and n-octadecane eutectic mixture with polystyrene shell for low-temperature latent heat thermal energy storage applications. Sol. Energy 2015, 115, 195–203. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Kızıl, Ç.; Bicer, A.; Sarı, A.; Alkan, C. Microencapsulated n-alkane eutectics in polystyrene for solar thermal applications. Sol. Energy 2018, 160, 32–42. [Google Scholar] [CrossRef]
- Döğüşcü, D.K.; Altıntaş, A.; Sarı, A.; Alkan, C. Polystyrene microcapsules with palmitic-capric acid eutectic mixture as building thermal energy storage materials. Energy Build. 2017, 150, 376–382. [Google Scholar] [CrossRef]
- Sami, S.; Sadrameli, S.; Etesami, N. Thermal properties optimization of microencapsulated a renewable and non-toxic phase change material with a polystyrene shell for thermal energy storage systems. Appl. Therm. Eng. 2018, 130, 1416–1424. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, H.; Wan, W.; Gao, X.; Zhang, Z. Preparation and thermal performance of polystyrene/n-tetradecane composite nanoencapsulated cold energy storage phase change materials. Energy Convers. Manag. 2013, 76, 430–436. [Google Scholar] [CrossRef]
- Irani, F.; Ranjbar, Z.; Moradian, S.; Jannesari, A. Microencapsulation of n-heptadecane phase change material with starch shell. Prog. Org. Coat. 2017, 113, 31–38. [Google Scholar] [CrossRef]
- Fang, Y.; Wei, H.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Preparation and thermal performance of silica/n-tetradecane microencapsulated phase change material for cold energy storage. Energy Fuels 2016, 30, 9652–9657. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, K.; Ou, X.; Xu, P.; Cheng, Y. Process and performance of palmitic acid@silica phase-change microcapsules using chemical precipitation method. J. Appl. Polym. Sci. 2022, 139, 51962. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y.; Gao, L. Fabrication and properties of microencapsulated paraffin@ SiO2 phase change composite for thermal energy storage. ACS Sustain. Chem. Eng. 2013, 1, 374–380. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Zhang, X.; Zhang, J.; Zhang, Z.; Yang, L. Synthesis and characterization of stearic acid/silicon dioxide nanoencapsules for solar energy storage. Sol. Energy 2018, 173, 42–52. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D.J. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Determining influences of SiO2 encapsulation on thermal energy storage properties of different phase change materials. Sol. Energy Mater. Sol. Cells 2017, 159, 1–7. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Qu, Z.; Ren, J.; Xiong, C. Microencapsulated capric–stearic acid with silica shell as a novel phase change material for thermal energy storage. Appl. Therm. Eng. 2014, 70, 546–551. [Google Scholar] [CrossRef]
- Tang, F.; Liu, L.; Alva, G.; Jia, Y.; Fang, G. Synthesis and properties of microencapsulated octadecane with silica shell as shape–stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 160, 1–6. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Chen, K.; Gao, X.; Chang, P.; Tian, C.; Wang, J.; Huang, Y. Preparation and properties of nanoencapsulated n-octadecane phase change material with organosilica shell for thermal energy storage. Energy Convers. Manag. 2015, 105, 908–917. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, S.; Wang, H.; Zhang, K.; Jia, X.; Tian, C.; Zhou, Y.; Wang, J. Morphological control and thermal properties of nanoencapsulated n-octadecane phase change material with organosilica shell materials. Energy Convers. Manag. 2016, 119, 151–162. [Google Scholar] [CrossRef]
- Pourmohamadian, H.; Sheikhzadeh, G.A.; Rahimi-Nasrabadi, M.; Tabrizi, H.B. Fabrication and characterization of microencapsulated PA with SiO2 shell through sol–gel synthesis via sodium silicate precursor. J. Mater. Sci. Mater. Electron. 2017, 28, 9990–9997. [Google Scholar] [CrossRef]
- Geng, L.; Wang, S.; Wang, T.; Luo, R. Facile synthesis and thermal properties of nanoencapsulated n-dodecanol with SiO2 shell as shape-formed thermal energy storage material. Energy Fuels 2016, 30, 6153–6160. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-change characteristics and thermal performance of form-stable n-alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- Pethurajan, V.; Sivan, S.; Konatt, A.J.; Reddy, A.S. Facile approach to improve solar thermal energy storage efficiency using encapsulated sugar alcohol based phase change material. Sol. Energy Mater. Sol. Cells 2018, 185, 524–535. [Google Scholar] [CrossRef]
- Deng, H.; Yang, Y.; Tang, X.; Li, Y.; He, F.; Zhang, Q.; Huang, Z.; Yang, Z.; Yang, W. Interfaces. Phase-change composites composed of silicone rubber and Pa@ SiO2@ PDA double-shelled microcapsules with low leakage rate and improved mechanical strength. ACS Appl. Mater. Interfaces 2021, 13, 39394–39403. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yang, W.; Cai, T.; He, F.; Li, Y.; Zhang, K.; He, R. Phase-change composites silicone rubber/paraffin@ SiO2 microcapsules with different core/shell ratio for thermal management. Int. J. Energy Res. 2021, 45, 18033–18047. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. fuels. Self-assembly synthesis of microencapsulated n-eicosane phase-change materials with crystalline-phase-controllable calcium carbonate shell. Energy Fuels 2014, 28, 3519–3529. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, T.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Engineering. Self-assembly synthesis and properties of microencapsulated n-tetradecane phase change materials with a calcium carbonate shell for cold energy storage. ACS Sustain. Chem. Eng. 2017, 5, 3074–3080. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Microencapsulated paraffin phase-change material with calcium carbonate shell for thermal energy storage and solar-thermal conversion. Langmuir 2018, 34, 14254–14264. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Geng, L.; Fang, Y. Enhancement on thermal properties of paraffin/calcium carbonate phase change microcapsules with carbon network. Appl. Energy 2016, 179, 601–608. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Guo, Y.; Jiang, Z.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Novel segregated-structure phase change materials composed of paraffin@ graphene microencapsules with high latent heat and thermal conductivity. J. Mater. Sci. 2018, 53, 2566–2575. [Google Scholar] [CrossRef]
- Dao, T.D.; Jeong, H.M. Novel stearic acid/graphene core–shell composite microcapsule as a phase change material exhibiting high shape stability and performance. Sol. Energy Mater. Sol. Cells 2015, 137, 227–234. [Google Scholar] [CrossRef]
- Advincula, P.; De Leon, A.; Rodier, B.; Kwon, J.; Advincula, R.; Pentzer, E. Accommodating volume change and imparting thermal conductivity by encapsulation of phase change materials in carbon nanoparticles. J. Mater. Chem. A 2018, 6, 2461–2467. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.; Wu, D. Development of bifunctional microencapsulated phase change materials with crystalline titanium dioxide shell for latent-heat storage and photocatalytic effectiveness. Appl. Energy 2015, 138, 661–674. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wu, D. Fabrication of microencapsulated phase change materials with TiO2/Fe3O4 hybrid shell as thermoregulatory enzyme carriers: A novel design of applied energy microsystem for bioapplications. Appl. Energy 2017, 201, 20–33. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D.; Ji, S. Fabrication and applications of dual-responsive microencapsulated phase change material with enhanced solar energy-storage and solar photocatalytic effectiveness. Sol. Energy Mater. Sol. Cells 2019, 193, 184–197. [Google Scholar] [CrossRef]
- Hou, M.; Jiang, Z.; Chu, F.; Zhang, X.; Lai, N.-C. N-eicosane@ TiO2/TiN composite phase change microcapsules: Efficient visible light-driven reversible solid-liquid phase transition. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129674. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Liu, P.; Gao, H.; Chang, Y.; Wang, G. Smart utilization of multifunctional metal oxides in phase change materials. Matter 2020, 3, 708–741. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, K.; He, R.; Yang, A.; Su, L.; Li, Y.; He, F.; Jiang, S.; Yang, W. Phase change composites based on double-shell microcapsules with high latent heat and low leakage rate for thermal energy storage and temperature regulation. J. Energy Storage 2022, 55, 105428. [Google Scholar] [CrossRef]
- Yin, D.; Liu, H.; Ma, L.; Zhang, Q. Fabrication and performance of microencapsulated phase change materials with hybrid shell by in situ polymerization in Pickering emulsion. Polym. Adv. Technol. 2015, 26, 613–619. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and performances of phase change materials microcapsules with a polymer/BN/TiO2 hybrid shell for thermal energy storage. Energy Fuels 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- Qiu, X.Z.; Tao, Y.; Xu, X.Q.; He, X.H.; Fu, X.Y. Synthesis and characterization of paraffin/TiO2-P (MMA-co-BA) phase change material microcapsules for thermal energy storage. J. Appl. Polym. Sci. 2018, 135, 46447. [Google Scholar] [CrossRef]
- Zhao, A.; An, J.; Yang, J.; Yang, E.-H. Microencapsulated phase change materials with composite titania-polyurea (TiO2-PUA) shell. Appl. Energy 2018, 215, 468–478. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, T.; Luo, G.; Zheng, B.; Huang, H.; Han, X.; Chai, Y. Facile method of fabricating microencapsulated phase change materials with compact bonding polymer–silica hybrid shell using TEOS/MPS. Thermochim. Acta 2018, 659, 183–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, H.; Du, Q. Encapsulated phase change materials stabilized by modified graphene oxide. J. Mater. Chem. A 2014, 2, 5304–5314. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, K.; Chen, Y.; Liu, R.; Luo, J. The preparation of linseed oil loaded graphene/polyaniline microcapsule via emulsion template method for self-healing anticorrosion coatings. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129771. [Google Scholar] [CrossRef]
- Shchukina, E.; Graham, M.; Zheng, Z.; Shchukin, D. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. R. Soc. Chem. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [Green Version]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Borreguero, A.; Valverde, J.; Rodríguez, J.; Barber, A.; Cubillo, J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm® RT27 obtained by spray drying. Chem. Eng. J. 2011, 166, 384–390. [Google Scholar] [CrossRef]
- Milián, Y.E.; Gutierrez, A.; Grageda, M.; Ushak, S. A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renew. Sustain. Energy Rev. 2017, 73, 983–999. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Li, M.; Ren, Y.; Xie, G. Ultralow friction polymer composites containing highly dispersed and thermally robust microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127989. [Google Scholar] [CrossRef]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Srinivasaraonaik, B.; Singh, L.; Tyangi, I.; Rawat, A.; Sinha, S. Microencapsulation of a eutectic PCM using in situ polymerization technique for thermal energy storage. Int. J. Energy Res. 2020, 44, 3854–3864. [Google Scholar]
- Wang, H.; Zhao, L.; Song, G.; Tang, G.; Shi, X. Organic-inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized pickering emulsion polymerization. Sol. Energy Mater. Sol. Cells 2018, 175, 102–110. [Google Scholar] [CrossRef]
- Chen, Q.; Ni, L.; Jiang, J.; Parker, T.; Chen, Z.; Cheng, Z.; Jiang, W.; Wang, Q. Inhibition of exothermic runaway of batch reactors for the homogeneous esterification using nano-encapsulated phase change materials. Appl. Therm. Eng. 2020, 178, 115531. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Silva, L.; Rodríguez, J.F.; Sánchez, P. Influence of different suspension stabilizers on the preparation of Rubitherm RT31 microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 62–66. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Designing behenic acid microcapsules as novel phase change material for thermal energy storage applications at medium temperature. Int. J. Energy Res. 2020, 44, 3922–3933. [Google Scholar] [CrossRef]
- Puupponen, S.; Seppälä, A.; Vartia, O.; Saari, K.; Ala-Nissilä, T. Preparation of paraffin and fatty acid phase changing nanoemulsions for heat transfer. Thermochim. Acta 2015, 601, 33–38. [Google Scholar] [CrossRef]
- Bakeshlou, Z.; Nikfarjam, N. Thermoregulating Papers Containing Fabricated Microencapsulated Phase Change Materials through Pickering Emulsion Templating. Ind. Eng. Chem. Res. 2020, 59, 20253–20268. [Google Scholar] [CrossRef]
- Onder, E.; Sarier, N.; Cimen, E. Encapsulation of phase change materials by complex coacervation to improve thermal performances of woven fabrics. Thermochim. Acta 2008, 467, 63–72. [Google Scholar] [CrossRef]
- Konuklu, Y.; Unal, M.; Paksoy, H.O. Microencapsulation of caprylic acid with different wall materials as phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 120, 536–542. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Buildings. Synthesis and characterization of microencapsulated paraffin with titanium dioxide shell as shape-stabilized thermal energy storage materials in buildings. Energy Build. 2014, 72, 31–37. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Yan, J.; Hu, D.; Wang, Z.; Ma, W. Construction strategies and thermal energy storage applications of shape-stabilized phase change materials. J. Appl. Polym. Sci. 2022, 139, 51550. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Tsubota, M.; Okinaka, N.; Akiyama, T. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl. Therm. Eng. 2013, 61, 825–828. [Google Scholar] [CrossRef]
- Khodadadi, J.; Hosseinizadeh, S. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Fang, X.; Zhang, Z. Preparation of graphite nanoparticles-modified phase change microcapsules and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 159, 159–166. [Google Scholar] [CrossRef]
- Parvate, S.; Singh, J.; Vennapusa, J.R.; Dixit, P.; Chattopadhyay, S. Copper nanoparticles interlocked phase-change microcapsules for thermal buffering in packaging application. J. Ind. Eng. Chem. 2021, 102, 69–85. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhao, T. Fabrication and characterization of poly (melamine-formaldehyde)/silicon carbide hybrid microencapsulated phase change materials with enhanced thermal conductivity and light-heat performance. Sol. Energy Mater. Sol. Cells 2018, 183, 82–91. [Google Scholar] [CrossRef]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Lu, B.; Shi, Z.; Zhang, Y.; Zhao, H.; Wang, Z. Investigation of the charging and discharging performance of paraffin/nano-Fe3O4 composite phase change material in a shell and tube thermal energy storage unit. Energy Storage Sci. Technol. 2021, 10, 1709–1719. [Google Scholar]
- Sun, Y.; Wang, R.; Liu, X.; Li, M.; Yang, H.; Li, B. Improvements in the thermal conductivity and mechanical properties of phase-change microcapsules with oxygen-plasma-modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2017, 134, 45269. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Yan, Y. Preparation and characteristics evaluation of mono and hybrid nano-enhanced phase change materials (NePCMs) for thermal management of microelectronics. Energy Convers. Manag. 2020, 205, 112444. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Wan, H.; Liu, H.; Wu, D.; Wang, X. Construction of polyaniline/carbon nanotubes-functionalized phase-change microcapsules for thermal management application of supercapacitors. Chem. Eng. J. 2020, 396, 125317. [Google Scholar] [CrossRef]
- Meng, X.; Qin, S.; Fan, H.; Huang, Z.; Hong, J.; Xu, X.; Ouyang, X.; Chen, D.-Z. Long alkyl chain-grafted carbon nanotube-decorated binary-core phase-change microcapsules for heat energy storage: Synthesis and thermal properties. Sol. Energy Mater. Sol. Cells 2020, 212, 110589. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, J.; Yu, S.; Wang, G. Improving thermal conductivity and decreasing supercooling of paraffin phase change materials by n-octadecylamine-functionalized multi-walled carbon nanotubes. RSC Adv. 2014, 4, 36584–36590. [Google Scholar] [CrossRef]
- Zeng, J.; Cao, Z.; Yang, D.; Sun, L.; Zhang, L. calorimetry. Thermal conductivity enhancement of Ag nanowires on an organic phase change material. J. Therm. Anal. Calorim. 2010, 101, 385–389. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.-Q.; Yu, Z.-T.; Xu, X.; Hu, Y.-C.; Cen, K.-F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Das, P.; Zheng, S.; Zhou, F.; Wu, Z.-S. Layer-by-layer stacked amorphous V2O5/Graphene 2D heterostructures with strong-coupling effect for high-capacity aqueous zinc-ion batteries with ultra-long cycle life. Energy Storage Mater. 2020, 31, 156–163. [Google Scholar] [CrossRef]
- Shi, X.; Tian, L.; Wang, S.; Wen, P.; Su, M.; Xiao, H.; Das, P.; Zhou, F.; Liu, Z.; Sun, C.J. Scalable and fast fabrication of graphene integrated micro-supercapacitors with remarkable volumetric capacitance and flexibility through continuous centrifugal coating. J. Energy Chem. 2021, 52, 284–290. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Yang, Q.; Li, X.; Li, H.; Wang, Y.; Jiao, T.; Huang, Z.; Dong, B.; Zhang, W. Highly Efficient Electrochemical Reduction of Nitrogen to Ammonia on Surface Termination Modified Ti3C2Tx MXene Nanosheets. ACS Nano 2020, 14, 9089–9097. [Google Scholar] [CrossRef]
- Li, X.; Xie, D.; Park, H.; Zeng, T.H.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Kong, J.; Zhu, H. Anomalous behaviors of graphene transparent conductors in graphene–silicon heterojunction solar cells. Adv. Energy Mater. 2013, 3, 1029–1034. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The antibacterial applications of graphene and its derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.; Adamson, D.; Schniepp, H.; Chen, X.; Ruoff, R. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Fang, X.; Fan, L.-W.; Wang, X.; Xiao, Y.-Q.; Zeng, Y.; Xu, X.; Hu, Y.-C.; Cen, K.-F. Increased thermal conductivity of liquid paraffin-based suspensions in the presence of carbon nano-additives of various sizes and shapes. Carbon 2013, 53, 277–285. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Arjmand, M. Graphene-based phase change composites for energy harvesting and storage: State of the art and future prospects. Carbon 2019, 148, 441–480. [Google Scholar] [CrossRef]
- Dao, T.D.; Jeong, H.M. A Pickering emulsion route to a stearic acid/graphene core–shell composite phase change material. Carbon 2016, 99, 49–57. [Google Scholar] [CrossRef]
- Wei, H.; Yang, W.; He, F.; Li, Y.; Lou, L.; Wang, R.; He, R.; Fan, J.; Zhang, K. Core@ double-shell structured multifunctional phase change microcapsules based on modified graphene oxide Pickering emulsion. Int. J. Energy Res. 2021, 45, 3257–3268. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Microencapsulated phase change material modified by graphene oxide with different degrees of oxidation for solar energy storage. Sol. Energy Mater. Sol. Cells 2018, 174, 453–459. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Qin, S.-Y.; Tsui, G.C.; Tang, C.-y.; Ouyang, X.; Liu, J.-h.; Tang, J.-N.; Zuo, J.-D. Fabrication, morphology and thermal properties of octadecylamine-grafted graphene oxide-modified phase-change microcapsules for thermal energy storage. Compos. Part B Eng. 2019, 157, 239–247. [Google Scholar] [CrossRef]
- Nurmawati, M.; Siow, K.; Rasiah, I.J. Characterization. Analysis of phase change material for use as thermal interface material. Int. J. Polym. Anal. Charact. 2004, 9, 213–228. [Google Scholar] [CrossRef]
- Shahid, U.B.; Abdala, A. A critical review of phase change material composite performance through Figure-of-Merit analysis: Graphene vs Boron Nitride. Energy Storage Mater. 2021, 34, 365–387. [Google Scholar] [CrossRef]
- Hu, D.; Liu, H.; Ding, Y.; Ma, W. Synergetic integration of thermal conductivity and flame resistance in nacre-like nanocellulose composites. Carbohydr. Polym. 2021, 264, 118058. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ma, W.; Zhang, Z.; Ding, Y.; Wu, L. Dual bio-inspired design of highly thermally conductive and superhydrophobic nanocellulose composite films. ACS Appl. Mater. Interfaces 2020, 12, 11115–11125. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ju, H.; Kim, J. Filler orientation of boron nitride composite via external electric field for thermal conductivity enhancement. Ceram. Int. 2016, 42, 8657–8663. [Google Scholar] [CrossRef]
- Wilk, A.; Rutkowski, P.; Zientara, D.; Bućko, M.M. Aluminium oxynitride–hexagonal boron nitride composites with anisotropic properties. J. Eur. Ceram. Soc. 2016, 36, 2087–2092. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-scale fabrication of boron nitride nanosheets and their utilization in polymeric composites with improved thermal and mechanical properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Fan, D.; Feng, J.; Liu, J.; Gao, T.; Ye, Z.; Chen, M.; Lv, X. Hexagonal boron nitride nanosheets exfoliated by sodium hypochlorite ball mill and their potential application in catalysis. Ceram. Int. 2016, 42, 7155–7163. [Google Scholar] [CrossRef]
- He, X.; Zhang, L.; Li, C. PEG-based polyurethane/Paraffin@ SiO2/Boron nitride phase change composite with efficient thermal conductive pathways and superior mechanical property. Compos. Commun. 2021, 25, 100609. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Q.; Wu, M.; Du, P.; Liu, C.; Rao, Z. Thermal properties optimization of lauric acid as phase change material with modified boron nitride nanosheets-sodium sulfate for thermal energy storage. J. Energy Storage 2023, 61, 106781. [Google Scholar] [CrossRef]
- Liao, H.; Guo, S.; Liu, Y.; Wang, Q. Form-stable phase change composites with high thermal conductivity and adjustable thermal management capacity. Sol. Energy Mater. Sol. Cells 2021, 221, 110881. [Google Scholar] [CrossRef]
- Wu, H.; Hu, X.; Li, X.; Sheng, M.; Sheng, X.; Lu, X.; Qu, J. Manufacturing. Large-scale fabrication of flexible EPDM/MXene/PW phase change composites with excellent light-to-thermal conversion efficiency via water-assisted melt blending. Compos. Part A: Appl. Sci. Manuf. 2022, 152, 106713. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Hu, D.; Liu, H.; Ma, W. Rational design of nanohybrids for highly thermally conductive polymer composites. Compos. Commun. 2020, 21, 100427. [Google Scholar] [CrossRef]
- Xu, B.; Wang, B.; Zhang, C.; Zhou, J. Synthesis and light-heat conversion performance of hybrid particles decorated MWCNTs/paraffin phase change materials. Thermochim. Acta 2017, 652, 77–84. [Google Scholar] [CrossRef]
- Rabady, R.I.; Dua’a, S.M. Thermal conductivity enhancement of sodium thiosulfate pentahydrate by adding carbon nano-tubes/graphite nano-particles. J. Energy Storage 2020, 27, 101166. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yu, F. Enhanced thermal conductivity of microencapsulated phase change materials based on graphene oxide and carbon nanotube hybrid filler. Sol. Energy Mater. Sol. Cells 2019, 192, 72–80. [Google Scholar] [CrossRef]
- Hu, D.; Liu, H.; Yang, M.; Guo, Y.; Ma, W. Construction of boron nitride nanosheets-based nanohybrids by electrostatic self-assembly for highly thermally conductive composites. Adv. Compos. Hybrid Mater. 2022, 5, 3201–3211. [Google Scholar] [CrossRef]
- Zhan, W.; Zhao, Y.; Yuan, Y.; Yi, H.; Song, S. Development of 2D-Mt/SA/AgNPs microencapsulation phase change materials for solar energy storage with enhancement of thermal conductivity and latent heat capacity. Sol. Energy Mater. Sol. Cells 2019, 201, 110090. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, H.; Liu, J.; Fang, X.; Zhang, Z. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2015, 143, 29–37. [Google Scholar] [CrossRef]
- Wu, M.Q.; Wu, S.; Cai, Y.F.; Wang, R.Z.; Li, T.X. Form-stable phase change composites: Preparation, performance, and applications for thermal energy conversion, storage and management. Energy Storage Mater. 2021, 42, 380–417. [Google Scholar] [CrossRef]
- Yin, H.; Gao, X.; Ding, J.; Zhang, Z.; Fang, Y. Thermal management of electronic components with thermal adaptation composite material. Appl. Energy 2010, 87, 3784–3791. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Wang, X.; Wu, D. Smart design and construction of nanoflake-like MnO2/SiO2 hierarchical microcapsules containing phase change material for in-situ thermal management of supercapacitors. Energy Convers. Manag. 2018, 164, 311–328. [Google Scholar] [CrossRef]
- Wang, T.; Tong, J.; Li, X.; Wang, S.; Deng, J. Research on morphological control and temperature regulation of phase change microcapsules with binary cores for electronics thermal management. Thermochim. Acta 2021, 706, 179079. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, P.; Zhu, J. Thermal management of electronic devices using pin-fin based cascade microencapsulated PCM/expanded graphite composite. Int. J. Heat Mass Transf. 2020, 149, 119199. [Google Scholar] [CrossRef]
- Xu, R.; Xia, X.; Wang, W.; Yu, D. Infrared camouflage fabric prepared by paraffin phase change microcapsule with good thermal insulting properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124519. [Google Scholar] [CrossRef]
- Niu, S.; Cheng, J.; Zhao, Y.; Kang, M.; Liu, Y. Preparation and characterization of multifunctional phase change material microcapsules with modified carbon nanotubes for improving the thermal comfort level of buildings. Constr. Build. Mater. 2022, 347, 128628. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Y.; Ma, D.; Li, S.; Zhang, F.; Guan, Y.; Qu, W.; Jin, Y.; Wang, D. Preparation and characterization of carbon nanotube microcapsule phase change materials for improving thermal comfort level of buildings. Constr. Build. Mater. 2020, 244, 118388. [Google Scholar] [CrossRef]
- Niu, Z.; Qi, S.; Shuaib, S.S.A.; Yuan, W. Flexible, stimuli-responsive and self-cleaning phase change fiber for thermal energy storage and smart textiles. Compos. Part B Eng. 2022, 228, 109431. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; He, Y. Novel smart textile with ultraviolet shielding and thermo-regulation fabricated via electrospinning. J. Energy Storage 2021, 42, 103094. [Google Scholar] [CrossRef]
- Kizildag, N. Smart composite nanofiber mats with thermal management functionality. Sci. Rep. 2021, 11, 4256. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; DeWitt, S.J.A.; McGuire, S.; Lively, R.P. Incorporation of Phase Change Materials into Fibers for Sustainable Thermal Energy Storage. Ind. Eng. Chem. Res. 2021, 60, 3374–3384. [Google Scholar] [CrossRef]
- Nejman, A.; Cieślak, M.; Gajdzicki, B.; Goetzendorf-Grabowska, B.; Karaszewska, A. Methods of PCM microcapsules application and the thermal properties of modified knitted fabric. Thermochim. Acta 2014, 589, 158–163. [Google Scholar] [CrossRef]
- Alay Aksoy, S.; Alkan, C.; Tözüm, M.S.; Demirbağ, S.; Altun Anayurt, R.; Ulcay, Y. Preparation and textile application of poly (methyl methacrylate-co-methacrylic acid)/n-octadecane and n-eicosane microcapsules. J. Text. Inst. 2017, 108, 30–41. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Wang, H.; Lin, P.; Jia, L.; Li, L.; Chen, Y. Synthesis and properties of multifunctional microencapsulated phase change material for intelligent textiles. J. Mater. Sci. 2021, 56, 2176–2191. [Google Scholar] [CrossRef]
- Chen, M.; Qian, Z.; Liu, H.; Wang, X. Size-tunable CaCO3@ n-eicosane phase-change microcapsules for thermal energy storage. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128470. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellon, C.; Nogues, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Al-Shannaq, R.; Fernández, A.I.; Farid, M.M. Preparation and characterization of microencapsulated phase change materials for use in building applications. Materials 2015, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, X.; Li, J.; Ma, C.; Guo, L.; Meng, X. Solar-driven phase change microencapsulation with efficient Ti4O7 nanoconverter for latent heat storage. Nano Energy 2018, 53, 579–586. [Google Scholar] [CrossRef]
- Liu, H.; Tian, X.; Ouyang, M.; Wang, X.; Wu, D.; Wang, X. Microencapsulating n-docosane phase change material into CaCO3/ Fe3O4 composites for high-efficient utilization of solar photothermal energy. Renew. Energy 2021, 179, 47–64. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, D. Design and synthesis of multifunctional microencapsulated phase change materials with silver/silica double-layered shell for thermal energy storage, electrical conduction and antimicrobial effectiveness. Energy 2016, 111, 498–512. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, H.; Fu, B.; Ji, Y.; Su, F.; Wilson, C.J. Heat transfer analysis of flash evaporation with MEPCM. J. Therm. Sci. Eng. Appl. 2019, 11, 051016. [Google Scholar] [CrossRef]







| Type | PCMs | Advantages | Disadvantages | Melting Point (°C) |
|---|---|---|---|---|
| Organic | Paraffin C16-C18 | Wide applicable temperature range Less supercooling phenomenon High crystallization rate High chemical stability Strong recycling performance Safety and non-toxic Little corrosion performance | Low thermal conductivity Poor thermal storage capacity per unit volume Volatilize easily Flammability Expensive | 22 |
| Paraffin C20-C33 | 50 | |||
| Stearic acid | 69 | |||
| Palmitic acid | 56 | |||
| Myristic acid | 58 | |||
| Lauric acid | 49 | |||
| Inorganic | MgSO4·7H2O | Lower volumetric expansion Large heat storage capacity Low cost High thermal conductivity Greater phase change enthalpy Non-flammable With a clear melting point Recyclable | High supercooling Prone to precipitation Poor dimensional stability Low thermal stability Corrosive Phase segregation Poor compatibility with some building materials | 49 |
| CaCl2·6H2O | 33 | |||
| Ba(OH)2·8H2O | 78 | |||
| Al(OH3)2·7H2O | 75 | |||
| KNO3 | 340 | |||
| Na2CO3 | 850 | |||
| Eutectic | CO(NH2)2-NH4Br | High thermal conductivity Hardly segregation Hardly supercooling | Expensive Strong odor | 80 |
| Na(CH3COO)·3H2O-CO(NH2)2 | 30 | |||
| NaCO3-LiCO3 | 490 | |||
| NaF-MgF2 | 640 |
| Shell Type | Shell Materials | Core Materials | Encapsulation Methods | Phase Change Enthalpy (J/g) | Encapsulation Rate (%) | Refs. |
|---|---|---|---|---|---|---|
| Organic shells | MF | Lauryl alcohol | In-situ polymerization | 187.5 | 93.1 | [45] |
| UF | Paraffins | In-situ polymerization | 72.4 | 52.8 | [49] | |
| PU | Methyl laurate | Interfacial polymerization | 136.2 | - | [63] | |
| PMMA | Na2HPO4·12H2O | Solvent evaporation | 142.9 | 80.4 | [58] | |
| Polyurea | CaCl2·6H2O | Interfacial polymerization | 118.3 | 71 | [60] | |
| PS | N-tetradecane | In-situ polymerization | 98.71 | 44.7 | [70] | |
| Starch | N-heptadecane | Interfacial polymerization | 187.27 | 78.27 | [71] | |
| Inorganic shells | SiO2 | N-octadecane | Interfacial polymerization | 109.5 | 51.5 | [73] |
| Mannitol | Sol-gel | 252.66 | 89.6 | [86] | ||
| CaCO3 | N-tetradecane | Self-assembly | 58.54 | 25.86 | [91] | |
| rGO | SA | Pickering emulsion polymerization | 159 | 74.3 | [96] | |
| TiO2 | N-eicosane | Interfacial polymerization | 188.27 | - | [99] | |
| Organic-inorganic hybrid shells | MF-SiO2 | N-hexadecanol | Pickering emulsion polymerization | 163.76 | 74.6 | [103] |
| PMMA-BN/TiO2 | Paraffin | Pickering emulsion polymerization | 124.4 | 72.1 | [104] | |
| P(MMA-co-BA)-TiO2 | Paraffin | Pickering emulsion polymerization | 90.12 | 36.09 | [105] | |
| PUA-TiO2 | N-octadecane | Interfacial polymerization | 181.1 | 77.3 | [106] | |
| PS-GO | N-hexadecane | Pickering emulsion polymerization | 186.8 | 78.5 | [108] |
| Types | Methods | Advantages | Disadvantages | Scope of Applications |
|---|---|---|---|---|
| Physical method | Spray drying | Simple operation High production efficiency Wide range of sizes | Low packaging rate Not used for inorganic PCMs | Organic PCMs Heat sensitive material |
| Solvent evaporation | Low cost | Low encapsulation efficiency | Inorganic PCMs | |
| Chemical method | In-situ polymerization | High encapsulation efficiencyStable shape Uniform coating | Complex operation Harmful for the environment | Organic PCMs Inorganic PCMs Organic shell material such as MF and UF |
| Interfacial polymerization | High reaction speed Simple operation Low permeability | The monomer is required to have a high reactivity Harmful for the environment | Organic PCMs Inorganic PCMs Organic shell material such as UF | |
| Suspension polymerization | Environmentally friendly Facile reaction condition High packaging rate | High energy consumption Expensive Not used for inorganic PCMs | Organic PCMs Large grained Organic shell material such as PMMA | |
| Emulsion polymerization | Stable High packaging rate High preparation efficiency Environmentally friendly | Complicated Expensive | Organic PCMs Inorganic PCMs Organic shell material such as PMMA Polymer/inorganic hybrid shell | |
| Physical-chemical method | Coacervation | Simple equipment Good control of the particle size and thickness | Difficult to scale-up Not used for inorganic PCMs Agglomeration | Organic PCMs Organic shell material |
| Sol-gel method | High thermal stability Strong controllability | Non-insulated and limited in building applications | Organic PCMs Inorganic PCMs Inorganic shell material |
| Dimensional | Thermally Conductive Fillers | MEPCMs | Thermal Conductivity (W/(m·K)) | Refs. | |
|---|---|---|---|---|---|
| Zero-dimensional | Graphite nanoparticles | Paraffin@MF/graphite nanoparticles | 0.312 | [134] | |
| Copper nanoparticles | Hexadecane@Copper nanoparticles interlocking polydivinylbenzene | 0.5045 | [135] | ||
| SiC nanoparticles | N-octadecane@PMF/SiC | 0.21 | [136] | ||
| Ag nanoparticles | N-OD@Silica/PDV/Ag | 1.346 | [137] | ||
| Fe3O4 nanoparticles | Paraffin@Fe3O4 nanoparticles | - | [138] | ||
| One-dimensional | CNTs | Paraffin@MWCNTs | 0.355 | [140] | |
| N-docosane@SiO2/PANi/CNTs | 0.846 | [141] | |||
| C18/C28@MF/OI modified CNTs | 0.329 | [142] | |||
| AgNWs | 1-Tetradecanol@AgNWs | 1.46 | [144] | ||
| Two-dimensional | Graphene | Paraffin@GNPs | 0.378 | [140] | |
| N-hexadecane@PS/GO | - | [108] | |||
| SA@Graphene | 0.352 | [155] | |||
| Paraffin@GO/PbWO4 | 0.735 | [156] | |||
| Dodecanol@MF/GO | 0.2790 | [157] | |||
| BN | Paraffin@SiO2/BN | 0.675 | [167] | ||
| N-octadecane@BN/MF | 0.184 | [12] | |||
| SA@SiO2/m-BN | 0.506 | [169] | |||
| BPs | Eicosane@PMMA/mBPs | - | [13] | ||
| Low-dimensional hybrids | “Point-line” structure | Cu2O/Cu/MWCNTs | Paraffin@Cu2O/Cu/MWCNTs | 0.43 | [173] |
| Graphite nanoparticles/CNTs | Sodium Thiosulfate Pentahydrate@CNTs/Graphite nanoparticles | 4.031 | [174] | ||
| “Point-plane” structure | TiO2 nanoparticles/BN | Paraffi@PMMA/BN/TiO2 | 0.4215 | [104] | |
| AgNPs/2D-Mt | SA@2D-Mt/AgNPs | 0.8223 | [177] | ||
| SiO2/GO | Paraffin@SiO2/GO | 1.162 | [178] | ||
| “Line-plane” structure | CNTs/GO | Dodecanol@MF/GO/CNTs | 0.3821 | [175] | |
| MWCNTs/GNPs | Paraffin@MWCNTs/GNPs | 0.430 | [140] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Tu, S.; Chen, J.; Zhang, H.; Chen, W.; Hu, D.; Lin, J. Phase Change Composite Microcapsules with Low-Dimensional Thermally Conductive Nanofillers: Preparation, Performance, and Applications. Polymers 2023, 15, 1562. https://doi.org/10.3390/polym15061562
Yang D, Tu S, Chen J, Zhang H, Chen W, Hu D, Lin J. Phase Change Composite Microcapsules with Low-Dimensional Thermally Conductive Nanofillers: Preparation, Performance, and Applications. Polymers. 2023; 15(6):1562. https://doi.org/10.3390/polym15061562
Chicago/Turabian StyleYang, Danni, Sifan Tu, Jiandong Chen, Haichen Zhang, Wanjuan Chen, Dechao Hu, and Jing Lin. 2023. "Phase Change Composite Microcapsules with Low-Dimensional Thermally Conductive Nanofillers: Preparation, Performance, and Applications" Polymers 15, no. 6: 1562. https://doi.org/10.3390/polym15061562