Preparation and Characterization of a New Bis-Uracil Chitosan-Based Hydrogel as Efficient Adsorbent for Removal of Anionic Congo Red Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
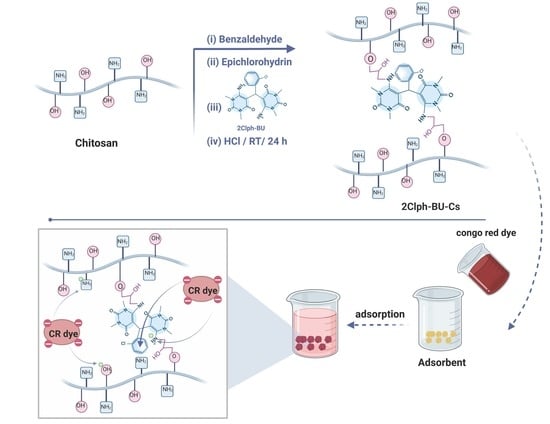
2.2.1. Synthesis of 2Clph-BU
2.2.2. Synthesis of a New 2Clph-BU-Cs Adsorbent
2.3. Measurements
2.3.1. Elemental Analysis
2.3.2. FTIR Spectroscopy
2.3.3. X-ray Diffractometry
2.3.4. Scanning Electron Microscopy
2.3.5. Swelling Capability Study
2.3.6. pH of Zero-Point Charge—pHzpc Study
2.4. Adsorption Behaviors
2.4.1. Adsorption Capacity
2.4.2. Adsorption Thermodynamics
2.4.3. Adsorption Kinetics
2.4.4. Adsorption Mechanism
2.4.5. Desorption Study
3. Results and Discussion
3.1. Synthesis of 2Clph-BU-Cs Adsorbent
3.2. Characterization of 2ClphBU-Cs Adsorbent
3.2.1. Elemental Analysis
3.2.2. FTIR Spectroscopy
3.2.3. Powder X-ray Diffractometry
3.2.4. SEM Analysis
3.2.5. pH of Zero-Point Charge (pHzpc)
3.2.6. Swelling Behavior of 2ClphBU-Cs
3.2.7. Factors Affecting the Adsorption Process
3.2.8. Adsorption Thermodynamics
3.2.9. Adsorption Kinetics
3.2.10. Adsorption Mechanism
3.2.11. Comparison between 2Clph-BU-Cs and Other Adsorbents to Remove CR dye
3.2.12. Desorption and Regeneration Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.; Batool, M.; Naz, F.; Nazar, M.F.; Hameed, B.H.; Zafar, M.N. A Comprehensive Review on Application of Plant-Based Bioadsorbents for Congo Red Removal. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Enhancement of Adsorption of Congo Red Dye onto Novel Antimicrobial Trimellitic Anhydride Isothiocyanate-Cross-Linked Chitosan Hydrogels. Polym. Bull. 2020, 77, 6135–6160. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Synthesis and Characterization of Novel Uracil-Modified Chitosan as a Promising Adsorbent for Efficient Removal of Congo Red Dye. Polymers 2022, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ouachtak, H.; Akhouairi, S.; Ait Addi, A.; Ait Akbour, R.; Jada, A.; Douch, J.; Hamdani, M. Mobility and Retention of Phenolic Acids through a Goethite-Coated Quartz Sand Column. Colloids Surf. 2018, 546, 9–19. [Google Scholar] [CrossRef]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.A.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafid, N.; Addi, A.A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis Toward Hazardous Rhodamine B Dye from Water. Arab. J. Sci. Eng. 2023, 48, 169–179. [Google Scholar] [CrossRef]
- Ouachtak, H.; Akhouairi, S.; Haounati, R.; Addi, A.A.; Jada, A.; Taha, M.L.; Douch, J. 3,4-Dihydroxybenzoic Acid Removal from Water by Goethite Modified Natural Sand Column Fixed-Bed: Experimental Study and Mathematical Modeling. Desalin. Water Treat. 2020, 194, 439–449. [Google Scholar] [CrossRef]
- Tony, M.A. Low-Cost Adsorbents for Environmental Pollution Control: A Concise Systematic Review from the Prospective of Principles, Mechanism and Their Applications. J. Dispers. Sci. Technol. 2022, 43, 1612–1633. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel Crosslinked O-Cm-Chitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Effective Removal of Basic Red 12 Dye by Novel Antimicrobial Trimellitic Anhydride Isothiocyanate-Cross-Linked Chitosan Hydrogels. Polym. Polym. Compos. 2021, 29, S274–S287. [Google Scholar] [CrossRef]
- Alharbi, R.A.; Alminderej, F.M.; Al-harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Design, Synthesis, and Characterization of Novel Bis-Uracil Chitosan Hydrogels Modified with Zinc Oxide Nanoparticles for Boosting Their Antimicrobial Activity. Polymers 2023, 15, 980. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Bin Arifin, M.A.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- El-Harby, N.F.; Ibrahim, S.M.A.; Mohamed, N.A. Adsorption of Congo Red Dye onto Antimicrobial Terephthaloyl Thiourea Cross-Linked Chitosan Hydrogels. Water Sci. Technol. 2017, 76, 2719–2732. [Google Scholar] [CrossRef] [Green Version]
- Elmehbad, N.Y.; Mohamed, N.A. Designing, Preparation and Evaluation of the Antimicrobial Activity of Biomaterials Based on Chitosan Modified with Silver Nanoparticles. Int. J. Biol. Macromol. 2020, 151, 92–103. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A. Evaluation of the Antimicrobial and Anti-Biofilm Activity of Novel Salicylhydrazido Chitosan Derivatives Impregnated with Titanium Dioxide Nanoparticles. Int. J. Biol. Macromol. 2022, 205, 719–730. [Google Scholar] [CrossRef]
- Alnawmasi, J.S. Construction of Amino-Thiol Functionalized Ion-Imprinted Chitosan for Lead (II) Ion Removal. Carbohydr. Polym. 2023, 308, 120596. [Google Scholar] [CrossRef] [PubMed]
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A.; Abdel-aziz, M.M. Green synthesis of nano-silver/sodium alginate/carboxymethyl xanthan gum hydrogel and evaluation of its anti-inflammatory and anti-helicobacter pylori activity. Cellul. Chem. Technol. 2022, 56, 983–995. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, Characterization, and Antimicrobial Activity of Carboxymethyl Chitosan-Graft-Poly(N-Acryloyl,N′-Cyanoacetohydrazide) Copolymers. J. Carbohydr. Chem. 2012, 31, 220–240. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Abdallah, H.M.; Mohamed, N.A.; Mohamed, R.R. Synthesis, Characterization and Application of Biodegradable Crosslinked Carboxymethyl Chitosan/Poly(Vinyl Alcohol) Clay Nanocomposites. Mater. Sci. Eng. C 2015, 56, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Alfuraydi, R.T.; Alminderej, F.M.; Mohamed, N.A. Evaluation of Antimicrobial and Anti-Biofilm Formation Activities of Novel Poly(Vinyl Alcohol) Hydrogels Reinforced with Crosslinked Chitosan and Silver Nano-Particles. Polymers 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Synthesis and Characterization of Novel Trimellitic Anhydride Isothiocyanate-Cross Linked Chitosan Hydrogels Modified with Multi-Walled Carbon Nanotubes for Enhancement of Antimicrobial Activity. Int. J. Biol. Macromol. 2019, 132, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Elmehbad, N.Y.; Mohamed, N.A. Terephthalohydrazido Cross-Linked Chitosan Hydrogels: Synthesis, Characterization and Applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 969–982. [Google Scholar] [CrossRef]
- Alkabli, J. Progress in Preparation of Thiolated, Crosslinked, and Imino-Chitosan Derivatives Targeting Specific Applications. Eur. Polym. J. 2022, 165, 110998. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, Characterization, and Antimicrobial Activity of Chitosan Hydrazide Derivative. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 410–415. [Google Scholar] [CrossRef]
- Pałasz, A.; Ciez, D. In Search of Uracil Derivatives as Bioactive Agents. Uracils and Fused Uracils: Synthesis, Biological Activity and Applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Ghobadpoor, A.; Safdari, T. Preparation, Characterization and Utilization of a Novel Dicationic Molten Salt as Catalyst for the Synthesis of Bis(6-Amino-1,3-Dimethyluracil-5-Yl)Methanes. Res. Chem. Intermed. 2020, 46, 1319–1327. [Google Scholar] [CrossRef]
- Fathalla, M.; Lawrence, C.M.; Zhang, N.; Sessler, J.L.; Jayawickramarajah, J. Base-Pairing Mediated Non-Covalent Polymers. Chem. Soc. Rev. 2009, 38, 1608–1620. [Google Scholar] [CrossRef]
- Das, S.; Thakur, A.J. A Clean, Highly Efficient and One-Pot Green Synthesis of Aryl/Alkyl/Heteroaryl-Substituted Bis(6-Amino-1,3-Dimethyluracil-5-Yl)Methanes in Water. Eur. J. Org. Chem. 2011, 2011, 2301–2308. [Google Scholar] [CrossRef]
- Bardagí, J.I.; Rossi, R.A. Advances in the Synthesis of 5-and 6-Substituted Uracil Derivatives. Org. Prep. Proced. Int. 2009, 41, 479–514. [Google Scholar] [CrossRef] [Green Version]
- Abdou, W.M.; Fahmy, A.F.M.; Kamel, A.A. A Facile Synthesis of Pyrrolo-[3,2-d]Pyrimidines from 6-Azidouracils and Ylide Phosphoranes. Heteroat. Chem. 2002, 13, 357–365. [Google Scholar] [CrossRef]
- Yunusa, U.; Usman, B.; Bashir Ibrahim, M. Adsorptive Removal of Basic Dyes and Hexavalent Chromium from Synthetic Industrial Effluent: Adsorbent Screening, Kinetic and Thermodynamic Studies. Int. J. Eng. Manuf. 2020, 10, 54–74. [Google Scholar] [CrossRef]
- Shukla, K.; Verma, A.; Verma, L.; Rawat, S.; Singh, J. A Novel Approach to Utilize Used Disposable Paper Cups for the Development of Adsorbent and Its Application for the Malachite Green and Rhodamine-B Dyes Removal from Aqueous Solutions. Nat. Environ. Pollut. Technol. 2020, 19, 57–70. Available online: http://neptjournal.com/upload-images/(5)B-3615-ap.pdf (accessed on 1 March 2020).
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Z. Chem. Und Ind. Kolloide 1907, 2, 15. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Of the Adsorption of Gases. Section II. Kinetics and Energetics of Gas Adsorption. Introductory Paper to Section II. Trans. Faraday Soc. 1932, 28, 195–201. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Proc. USSR Acad. Sci. 1947, 55, 327–329. [Google Scholar]
- Singh, J.S. Vibrational Spectra of Bio-Molecules: Uracil. In Spectroscopy of Biological Molecules; Greve, J., Puppels, G.J., Otto, C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1999; p. 275. ISBN 978-94-011-4479-7. [Google Scholar]
- Wu, B.; Wang, Y.; Chen, S.; Wang, M.; Ma, M.; Shi, Y.; Wang, X. Bis-Uracil Based High Efficient Heat Stabilizers Used in Super Transparent Soft Poly (Vinyl Chloride). Polym. Degrad. Stab. 2018, 149, 143–151. [Google Scholar] [CrossRef]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of Crystalline Structural Differences Between α- and β-Chitosan on Their Nanoparticle Formation Via Ionic Gelation and Superoxide Radical Scavenging Activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.A.; El-Ghany, N.A.A. Swelling Behavior of Cross-Linked Terephthaloyl Thiourea Carboxymethyl Chitosan Hydrogels. Cellul. Chem. Technol. 2016, 50, 463–471. [Google Scholar]
- Xiao, D.; He, M.; Liu, Y.; Xiong, L.; Zhang, Q.; Wei, L.; Li, L.; Yu, X. Strong Alginate/Reduced Graphene Oxide Composite Hydrogels with Enhanced Dye Adsorption Performance. Polym. Bull. 2020, 77, 6609–6623. [Google Scholar] [CrossRef]
- Hu, X.S.; Liang, R.; Sun, G. Super-Adsorbent Hydrogel for Removal of Methylene Blue Dye from Aqueous Solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Mittal, H.; Morajkar, P.P.; Al Alili, A.; Alhassan, S.M. In-Situ Synthesis of ZnO Nanoparticles Using Gum Arabic Based Hydrogels as a Self-Template for Effective Malachite Green Dye Adsorption. J. Polym. Environ. 2020, 28, 1637–1653. [Google Scholar] [CrossRef]
- Zaheer, Z.; AbuBaker Bawazir, W.; Al-Bukhari, S.M.; Basaleh, A.S. Adsorption, Equilibrium Isotherm, and Thermodynamic Studies to the Removal of Acid Orange 7. Mater. Chem. Phys. 2019, 232, 109–120. [Google Scholar] [CrossRef]
- Ojedokun, A.T.; Bello, O.S. Liquid Phase Adsorption of Congo Red Dye on Functionalized Corn Cobs. J. Dispers. Sci. Technol. 2017, 38, 1285–1294. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, S.; Zhang, C.; Fu, Z.; Wang, A.; Zhang, Q.; Wang, Y.; Liu, X.; Wang, X.; Xu, W. Environment-Friendly Juncus Effusus-Based Adsorbent with a Three-Dimensional Network Structure for Highly Efficient Removal of Dyes from Wastewater. J. Clean. Prod. 2020, 259, 120812. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, Y.; Wang, X.; Wang, S.; Cheng, T.; Ping, R.; Cao, J.; Lv, K. Novel Chitosan and Laponite Based Nanocomposite for Fast Removal of Cd(II), Methylene Blue and Congo Red from Aqueous Solution. E-Polymers 2019, 19, 244–256. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y.; Li, J.; Zhang, Z.; Qiao, B.; Sui, K.; Wang, D.; Wang, C.; Li, H.; Xia, Y. Preparation of Graphene Oxide/Chitosan Pellets and Their Adsorption Properties for Congo Red. Int. J. Nanosci. 2019, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Li, R.; Miao, P.; Gao, J.; Hu, G.; Zhao, Y.; Chen, T. Design, Synthesis and Adsorption Evaluation of Bio-Based Lignin/Chitosan Beads for Congo Red Removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef] [PubMed]
- Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Al-Ola, K.A.A.; Hamd, A.; Soliman, N.K.; Ahmed, S.A. Design, Characterization, and Adsorption Properties of Padina Gymnospora/Zeolite Nanocomposite for Congo Red Dye Removal from Wastewater. Sci. Rep. 2021, 11, 21058. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, N. Equilibrium Studies on Sorption of an Anionic Dye onto Acid Activated Water Hyacinth Roots. Afr. J. Environ. Sci. Technol. 2009, 3, 399–404. [Google Scholar]

























| Sample Code | Elemental Analysis | ||||
|---|---|---|---|---|---|
| % C | % H | % N | % O | % Cl | |
| Cs | 44.90 | 6.86 | 8.61 | 39.63 | - |
| CsSB | 62.80 | 5.98 | 5.70 | 25.52 | - |
| ECsSB | 63.08 | 6.11 | 4.48 | 26.33 | - |
| 2Clph-BU-CsSB | 58.62 | 5.65 | 10.79 | 21.52 | 3.42 |
| 2Clph-BU-Cs | 51.14 | 5.83 | 13.00 | 25.80 | 4.23 |
| ΔH° (kJ mol−1) | ΔS° (J mol−1K−1) | ΔG° (kJ mol−1) | R2 (ln Kc against 1/T) | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | 333 K | |||
| 43.70 | 140.76 | 1.26 | 0.86 | −1.45 | −2.23 | −3.47 | 0.923 |
| Kinetic Models | Parameters | ||
|---|---|---|---|
| qe.exp (mg g−1) | 35.56 | ||
| pseudo- first-order | R2 | 0.942 | |
| qe.cal (mg g−1) | 0.15 | ||
| k1 (10−4)(min−1) | 16.1 | ||
| pseudo- second-order | R2 | 0.998 | |
| qe,cal (mg g−1) | 36.10 | ||
| k2 (10−4)(g mg−1 min−1) | 2.83 | ||
| Elovich | R2 | 0.976 | |
| β (g mg−1) | 0.21 | ||
| α (mg g−1 min−1) | 1.66 | ||
| Intraparticle Diffusion | 1st | Kint | 16.12 |
| R2 | 0.996 | ||
| 2nd | Kint | 0.36 | |
| R2 | 0.999 | ||
| 3rd | Kint | 0.01 | |
| R2 | 0.793 | ||
| Isotherm Mode | Parameters | 298 K | 308 K | l328 K |
|---|---|---|---|---|
| Langmuir | R2 | 0.985 | 0.996 | 0.999 |
| KL (L mg−1) | 0.01 | 0.01 | 1.11 | |
| qm | 49.75 | 93.46 | 59.52 | |
| RL | 0.48–0.77 | 0.44–0.74 | 0.41–0.63 | |
| Freundlich | R2 | 0.953 | 0.995 | 0.903 |
| Kf | 4.96 | 2.07 | 46.52 | |
| 1/n | 0.47 | 0.71 | 0.05 | |
| Temkin | R2 | 0.970 | 0.992 | 0.905 |
| KT (L mg−1) | 0.28 | 0.13 | 2,268,261 | |
| B (J mol−1) | 12.05 | 18.32 | 3.16 | |
| D-R | R2 | 0.896 | 0.924 | 0.937 |
| qm (mg g−1) | 34.11 | 37.72 | 57.39 | |
| (mol2 J−2) | 2 × 10−5 | 4 × 10−5 | 6 × 10−7 | |
| E (kJ mol−1) | 0.159 | 0.112 | 0.909 |
| Adsorbent | Removal Efficiency (%) | Temperature °C | Dye Concentration mg L−1 | Adsorbent Dose (g) | pH | Ref. |
|---|---|---|---|---|---|---|
| Chitosan and Laponite based nanocomposite | 78.06 | 30 | 500 | 1 | 6 | [50] |
| GO/Chitosan | 84.6 | 15 | 50 | 0.05 | - | [51] |
| Lignin/Chitosan | 86.5 | 35 | 200 | 0.01 | 3 | [52] |
| Padina, gymnospora Zeolite | 78.89 | 25 | 20 | 0.02 | 7 | [53] |
| Water hyacinth roots | 46.15 | 25 | 20 | 1 | 6 | [54] |
| 2Clph-BU-Cs | 95.73 | 55 | 50 | 0.01 | 4 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Preparation and Characterization of a New Bis-Uracil Chitosan-Based Hydrogel as Efficient Adsorbent for Removal of Anionic Congo Red Dye. Polymers 2023, 15, 1529. https://doi.org/10.3390/polym15061529
Alharbi RA, Alminderej FM, Al-Harby NF, Elmehbad NY, Mohamed NA. Preparation and Characterization of a New Bis-Uracil Chitosan-Based Hydrogel as Efficient Adsorbent for Removal of Anionic Congo Red Dye. Polymers. 2023; 15(6):1529. https://doi.org/10.3390/polym15061529
Chicago/Turabian StyleAlharbi, Rana A., Fahad M. Alminderej, Nouf F. Al-Harby, Noura Y. Elmehbad, and Nadia A. Mohamed. 2023. "Preparation and Characterization of a New Bis-Uracil Chitosan-Based Hydrogel as Efficient Adsorbent for Removal of Anionic Congo Red Dye" Polymers 15, no. 6: 1529. https://doi.org/10.3390/polym15061529