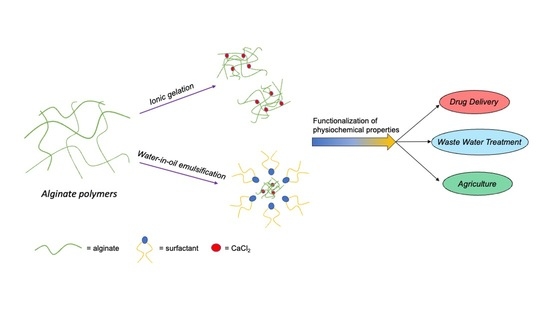
Synthesis of Alginate Nanoparticles Using Hydrolyzed and Enzyme-Digested Alginate Using the Ionic Gelation and Water-in-Oil Emulsion Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Breakdown of Alginate
2.2.1. Acid Hydrolysis
2.2.2. Alginate Lyase
2.2.3. Mass Spectrometry
2.3. Alginate Nanoparticle Synthesis and Characterization
2.3.1. Ionic Gelation
2.3.2. Water-in-Oil Emulsification
2.3.3. Alginate Nanoparticle Characterization
3. Results and Discussion
3.1. Alginate Hydrolysis
3.2. Alginate Nanoparticle Synthesis
3.2.1. Ionic Gelation
3.2.2. Water-in-Oil Emulsification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, P.; Daear, W.; Löbenberg, R.; Prenner, E.J. Overview of the Preparation of Organic Polymeric Nanoparticles for Drug Delivery Based on Gelatine, Chitosan, Poly(d,l-Lactide-Co-Glycolic Acid) and Polyalkylcyanoacrylate. Colloids Surf. B Biointerfaces 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A Comprehensive Review on Recent Advances in Preparation, Physicochemical Characterization, and Bioengineering Applications of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer Based Nanomaterials in Drug Delivery Systems: A Review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-Based Nanoparticles and Microparticles: Fabrication, Characterization, and Application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Dodero, A.; Donati, I.; Scarfì, S.; Mirata, S.; Alberti, S.; Lova, P.; Comoretto, D.; Alloisio, M.; Vicini, S.; Castellano, M. Effect of Sodium Alginate Molecular Structure on Electrospun Membrane Cell Adhesion. Mater. Sci. Eng. C 2021, 124, 112067. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveria, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Hu, X.; Feng, H.; Xiong, W.; Guo, W.; Zhou, J.; Mosa, A.; Peng, Y. Multicavity Triethylenetetramine-Chitosan/Alginate Composite Beads for Enhanced Cr(VI) Removal. J. Clean. Prod. 2019, 231, 733–745. [Google Scholar] [CrossRef]
- Bibi, A.; Rehman, S. Alginate-Nanoparticles Composites: Kinds, Reactions and Applications. Mater. Res. Express 2019, 6, 092001. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration Behaviors of Alginate Blocks at Various Calcium Concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef]
- Campos-Vallette, M.M.; Chandía, N.P.; Clavijo, E.; Leal, D.; Matsuhiro, B.; Osorio-Román, I.O.; Torres, S. Characterization of Sodium Alginate and Its Block Fractions by Surface-Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2010, 41, 758–763. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Krzan, M.; Grzyb, J. Synthesis of Silver and Gold Nanoparticles in Sodium Alginate Matrix Enriched with Graphene Oxide and Investigation of Properties of the Obtained Thin Films. Appl. Sci. 2021, 11, 3857. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B. A Study on the Constitution of Alginic Acid By Partial Acid Hydrolysis. In Proceedings of the Fifth International Seaweed Symposium, Halifax, UK, 25–28 August 1965; Elsevier: Amsterdam, The Netherlands, 1966; pp. 271–277. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Ochi, Y.; Kawabata, Y.; Kusakabe, I.; Takeuchi, T.; Murata, K. A Simple Method for Preparation of Poly-Mannuronate Using Poly-Guluronate Lyase. Biosci. Biotechnol. Biochem. 1995, 59, 1560–1561. [Google Scholar] [CrossRef]
- Zahran, M.K.; Ahmed, H.B.; El-Rafie, M.H. Alginate Mediate for Synthesis Controllable Sized AgNPs. Carbohydr. Polym. 2014, 111, 10–17. [Google Scholar] [CrossRef]
- Chandia, N.P.; Matsuhiro, B.; Vasquez, A.E. Alginic Acids in Lessonia Trabeculata: Characterization by Formic Acid Hydrolysis and FT-IR Spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Shi, J.; Zhu, R.; Zhang, J.; Zhang, Z.; Ma, D.; Hou, Y.; Lin, F.; Yang, J.; et al. A Biomimetic Silk Fibroin/Sodium Alginate Composite Scaffold for Soft Tissue Engineering. Sci. Rep. 2016, 6, 39477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamura, E.; Albanese, P.; Mavelli, F.; Stano, P. The Rise of the Nested Multicompartment Model in Synthetic Cell Research. Front. Mol. Biosci. 2021, 8, 750576. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Nguyen, O.T.; Oldenburg, S.J. Nanoparticle Characteristics Affecting Environmental Fate and Transport through Soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef]
- Christian, P.; Von Der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, Properties, Preparation and Behaviour in Environmental Media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Banerjee, J.; Das, B.; Mandal, J.; Chatterjee, S.; Ali, K.M.; Sinha, S.; Giri, B.; Ghosh, T.; Dash, S.K. Antibacterial Potency of Cytocompatible Chitosan-Decorated Biogenic Silver Nanoparticles and Molecular Insights towards Cell-Particle Interaction. Int. J. Biol. Macromol. 2022, 219, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of Polymeric Nanoparticle Size to Improve Therapeutic Delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Chia, J.J.; Shameli, K.; Yusefi, M.; Ali, R.R.; Balasundram, V.; Teow, S.Y. Preparation and Application of Cross-Linked Alginate Nanoparticles as Drug Carrier: A Review. J. Res. Nanosci. Nanotechnol. 2022, 5, 1–11. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Saraei, F.; Mohamadpour Dounighi, N.; Zolfagharian, H.; Moradi Bidhendi, S.; Khaki, P.; Inanlou, F. Design and Evaluate Alginate Nanoparticles as a Protein Delivery System. Arch. Razi Inst. 2013, 68, 139–146. [Google Scholar] [CrossRef]
- You, J.O.; Peng, C.A. Calcium-Alginate Nanoparticles Formed by Reverse Microemulsion as Gene Carriers. Macromol. Symp. 2005, 219, 147–153. [Google Scholar] [CrossRef]
- Ariyadasa, S.; Daear, W.; Abeysekera, G.; Billington, C.; Fee, C.; Prenner, E.; Pang, L. Evaluation of Biopolymer Materials and Synthesis Techniques to Develop a Rod-Shaped Biopolymer Surrogate for Legionella Pneumophila. Polymers 2022, 14, 2571. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Piao, Y.L.; Huang, X.; Yoon, E.J.; Park, S.H.; Lee, K.; Zhan, C.G.; Cho, H. Modeling and Re-Engineering of Azotobacter Vinelandii Alginate Lyase to Enhance Its Catalytic Efficiency for Accelerating Biofilm Degradation. PLoS ONE 2016, 11, e0156197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of Chitosan/Tripolyphosphate Nanoparticles with Highly Tunable Size and Low Polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lapitsky, Y. Salt-Assisted Mechanistic Analysis of Chitosan/Tripolyphosphate Micro- and Nanogel Formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef] [PubMed]
- Mesa, C.L.; Coppola, L.; Ranieri, G.A.; Terzeni, G.; Giuseppe, C. Phase Diagram and Phase Properties of the System Water-Hexane-Aerosol OT. Langmuir 1992, 8, 2616–2622. [Google Scholar] [CrossRef]
- Krishnakumar, S.; Somasundaran, P. Aggregation Behavior of Aerosol OT in Nonaqueous Solvents and Its Desorption—An ESR Study. J. Colloid Interface Sci. 1994, 162, 425–430. [Google Scholar] [CrossRef]








| Sample ID | Alginate Digest | Alginate (mg/mL) | CaCl2 (mg/mL) | Size (nm) % Population | PDI | Homogenization Method/Time |
|---|---|---|---|---|---|---|
| IG1 | Acid hydrolyzed | 0.095 | 0.10 | 227.6 ± 19.7; 100% | 0.178 | Magnetic stirring (1300 rpm, 45 min) |
| IG2 | Acid hydrolyzed | 0.285 | 0.10 | 242.2 ± 9.5; 100% | 0.155 | Magnetic stirring (1300 rpm, 45 min) |
| IG3 | Acid hydrolyzed | 0.570 | 0.10 | 206.6 ± 3.5; 100% | 0.163 | Magnetic stirring (1300 rpm, 45 min) |
| IG4 | Enzyme-digested | 0.095 | 0.03 | 125.9 ± 11.8; 100% | 0.617 | Magnetic stirring (1300 rpm, 45 min) |
| IG5 | Enzyme-digested | 0.095 | 0.10 | 118.3 ± 20.1; 100% | 0.695 | Magnetic stirring (1300 rpm, 45 min) |
| IG6 | Enzyme-digested | 0.095 | 0.55 | 232.2 ± 5.4; 100% | 0.072 | Magnetic stirring (1300 rpm, 45 min) |
| IG7 | Enzyme-digested | 0.285 | 0.10 | 200.9 ± 3.2; 100% | 0.17 | Magnetic stirring (1300 rpm, 45 min) |
| IG8 | Enzyme-digested | 0.285 | 0.20 | 571.9 ± 6.7; 100% | 0.49 | Magnetic stirring (1300 rpm, 45 min) |
| IG9 | Enzyme-digested | 0.285 | 0.40 | 2826 ± 22.8; 100% | 0.44 | Magnetic stirring (1300 rpm, 45 min) |
| IG10 | Enzyme-digested | 0.095 | 0.10 | 108.3 ± 2.5; 100% | 0.165 | Bath sonication (60 min) |
| IG11 | Enzyme-digested | 0.095 | 0.28 | 342.5 ± 1.9; 100% | 0.247 | Bath sonication (60 min) |
| IG12 | Enzyme-digested | 1.2 | 0.10 | 400.7 ± 4.8; 100% | 0.299 | Bath sonication (60 min) |
| IG13 | Enzyme-digested | 1.2 | 0.28 | 319.0 ± 3.1; 100% | 0.269 | Bath sonication (60 min) |
| IG14 | Enzyme-digested | 2.7 | 0.1 | 96.56 ± 5.9; 21.1% 722.1 ± 4.7; 41.2% | 0.927 | Homogenizer (7000 rpm, 35 min) |
| Sample ID | (Alginate) (mg/mL); (CaCl2) (mg/mL) | Size (nm); % Population | PDI | Washing Method |
|---|---|---|---|---|
| E1 | 0.5; 0.6 | 138.8 ± 13.2; 100% | 0.210 | N/A |
| E2 | 0.5; 0.6 | 455.3 ± 41.9; 100% | 0.492 | Acetone |
| E3 | 0.5; 0.6 | 625.5 ± 53.5; 100% | 0.423 | Isopropanol |
| E4 | 0.5; 0.6 | 220.6 ± 9.2; 100% | 1 | Acetone + 0.2 μm filter |
| E5 | 0.5; 0.6 | 426.6 ± 38.7; 33.7% | 1 | Acetone + ethyl acetate |
| E6 | 0.5; 0.6 | 141.7 ± 51.6; 22% | 1 | Acetone + ethyl acetate + 0.2 µm filter |
| E7 | 2.5; 0.6 | 144.3 ± 20.6; 76% | 1 | Acetone + ethyl acetate + 0.2 µm filter |
| E8 | 5.0; 0.6 | 945.8 ± 81.3; 32% | 1 | Acetone + ethyl acetate + 0.2 µm filter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Bavel, N.; Lewrenz, A.-M.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. Synthesis of Alginate Nanoparticles Using Hydrolyzed and Enzyme-Digested Alginate Using the Ionic Gelation and Water-in-Oil Emulsion Method. Polymers 2023, 15, 1319. https://doi.org/10.3390/polym15051319
Van Bavel N, Lewrenz A-M, Issler T, Pang L, Anikovskiy M, Prenner EJ. Synthesis of Alginate Nanoparticles Using Hydrolyzed and Enzyme-Digested Alginate Using the Ionic Gelation and Water-in-Oil Emulsion Method. Polymers. 2023; 15(5):1319. https://doi.org/10.3390/polym15051319
Chicago/Turabian StyleVan Bavel, Nicolas, Anna-Marie Lewrenz, Travis Issler, Liping Pang, Max Anikovskiy, and Elmar J. Prenner. 2023. "Synthesis of Alginate Nanoparticles Using Hydrolyzed and Enzyme-Digested Alginate Using the Ionic Gelation and Water-in-Oil Emulsion Method" Polymers 15, no. 5: 1319. https://doi.org/10.3390/polym15051319