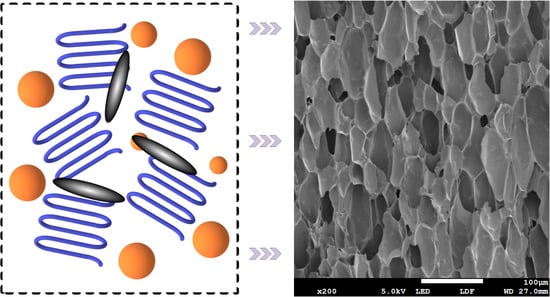
Refined Spherulites of PP Induced by Supercritical N2 and Graphite Nanosheet and Foaming Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Online Characterization of Crystallization Behavior
2.3. MOFIM Fabrication Process
2.4. Characterizations
3. Secondary Nucleation Model
4. Results and Discussions
4.1. Structure and Morphology
4.2. Crystalline Morphology of PP/GN
4.3. Secondary Nucleation Rate of PP/GN Nanocomposite
4.4. Foaming Performance of PP/GN Nanocomposite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, C.; Zhang, Q.; Zhang, W.; Xia, M.; Yan, K.; Lu, J.; Wu, G. High thermal insulation and compressive strength polypropylene microcellular foams with honeycomb structure. Polym. Degrad. Stab. 2020, 183, 109406. [Google Scholar] [CrossRef]
- Wu, G.; Xie, P.; Yang, H.; Dang, K.; Xu, Y.; Sain, M.; Turng, L.-S.; Yang, W. A review of thermoplastic polymer foams for functional applications. J. Mater. Sci. 2021, 56, 11579–11604. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Chen, Z.; Huang, Y.; Wang, C.; Zhang, A.; Park, C.B. Microcellular injection molded outstanding oleophilic and sound-insulating PP/PTFE nanocomposite foam. Compos. Part B Eng. 2021, 215, 108786. [Google Scholar] [CrossRef]
- Li, W.; Ren, Q.; Zhu, X.; Wu, M.; Weng, Z.; Wang, L.; Zheng, W. Enhanced heat resistance and compression strength of microcellular poly (lactic acid) foam by promoted stereocomplex crystallization with added D-Mannitol. J. CO2 Util. 2022, 63, 102118. [Google Scholar] [CrossRef]
- Jia, L.J.; Phule, A.D.; Geng, Y.; Wen, S.; Li, L.; Zhang, Z.X. Microcellular Conductive Carbon Black or Graphene/PVDF Composite Foam with 3D Conductive Channel: A Promising Lightweight, Heat-Insulating, and EMI-Shielding Material. Macromol. Mater. Eng. 2021, 306, 2000759. [Google Scholar] [CrossRef]
- Xu, M.; Wei, L.; Ma, L.; Lu, J.; Liu, T.; Zhang, L.; Zhao, L.; Park, C. Microcellular foamed polyamide 6/carbon nanotube composites with superior electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 117, 215–224. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, G.; Zhang, Y.; Fan, X.; Wang, Z.; Zhang, S.; Xiao, R.; Huang, F.; Shi, X.; Qin, J. Absorption dominated high-performance electromagnetic interference shielding epoxy/functionalized reduced graphene oxide/Ni-chains microcellular foam with asymmetric conductive structure. Compos. Sci. Technol. 2022, 223, 109419. [Google Scholar] [CrossRef]
- Lauritzen, I.; John, I.; Hoffman, J.D. Extension of theory of growth of chain-folded polymer crystals to large undercoolings. J. Appl. Phys. 1973, 44, 4340–4352. [Google Scholar] [CrossRef]
- Lindenmeyer, P.H. Surface area and secondary nucleation theory. Nature 1977, 269, 396–397. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular Physics; Academic Press: Cambridge, MA, USA, 1976; Volume 2. [Google Scholar]
- Pérez-Martín, H.; Mackenzie, P.; Baidak, A.; Brádaigh, C.M.; Ray, D. Crystallisation behaviour and morphological studies of PEKK and carbon fibre/PEKK composites. Compos. Part A Appl. Sci. Manuf. 2022, 159, 106992. [Google Scholar] [CrossRef]
- Liuyun, J.; Chengdong, X.; Lixin, J.; Lijuan, X. Effect of hydroxyapatite with different morphology on the crystallization behavior, mechanical property and in vitro degradation of hydroxyapatite/poly(lactic-co-glycolic) composite. Compos. Sci. Technol. 2014, 93, 61–67. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Wang, H.; Yu, Y. Non-isothermal crystallization kinetics of polypropylene/bamboo fiber/nano-TiO2 composites. Polym. Compos. 2021, 42, 2531–2543. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Chen, S.; Wang, X. The synergistic effect of polytetrafluoroethylene in-situ fibrillation and dibenzoyl sebacate hydrazide on the crystallization and foaming behavior of poly (lactic acid). Int. J. Biol. Macromol. 2022, 221, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, S.; Han, S.; Yu, K.; Wang, L. Regulating cell morphology of poly (lactic acid) foams from microcellular to nanocellular by crystal nucleating agent. Polym. Degrad. Stab. 2022, 204, 110117. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Xiang, A.; Ma, S.; Tian, H.; Ouyang, Y.; Rajulu, A.V. Crystallization behavior of polyvinyl alcohol with inorganic nucleating agent talc and regulation mechanism analysis. J. Polym. Environ. 2022, 30, 3163–3173. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J.; Zhou, J.; Nan, J. Nucleation effect of cellulose nanocrystals/polybutylene succinate composite filler on polylactic acid/polybutylene succinate blends. Polym. Bull. 2022, 79, 5481–5494. [Google Scholar] [CrossRef]
- Kulkarni, A. Effect of ionic interactions on crystallization of star telechelic poly(l-lactide) ionomers. Polymer 2022, 252, 124939. [Google Scholar] [CrossRef]
- Cherri, A.; Iliopoulos, I.; Régnier, G.; Brulé, B.; Lé, G.; Tencé-Girault, S. Thermal and Crystallization Properties of the Alternated Tere/Iso PEKK Copolymer: Importance in High-Temperature Laser Sintering. ACS Appl. Polym. Mater. 2022, 4, 2806–2818. [Google Scholar] [CrossRef]
- Toda, A. Effect of a Nucleating Agent on Polymer Crystallization Analyzed Using the Original Avrami Model. Macromolecules 2022, 55, 2202–2209. [Google Scholar] [CrossRef]
- Li, D.-C.; Liu, T.; Zhao, L.; Yuan, W.-K. Foaming of linear isotactic polypropylene based on its non-isothermal crystallization behaviors under compressed CO2. J. Supercrit. Fluids 2011, 60, 89–97. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Zhao, L. Direct melt-crystallization of isotactic poly-1-butene with form I′ using high-pressure CO2. Polymer 2011, 52, 5659–5668. [Google Scholar] [CrossRef]
- Nofar, M.; Tabatabaei, A.; Ameli, A.; Park, C.B. Comparison of melting and crystallization behaviors of polylactide under high-pressure CO2, N2, and He. Polymer 2013, 54, 6471–6478. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Li, B.; Li, H.; Cao, Z.; Jin, G.; Zhao, L.; Xin, Z. Foaming and dimensional stability of LDPE foams with N2, CO2, i-C4H10 and CO2—N2 mixtures as blowing agents. J. Supercrit. Fluids 2020, 164, 104930. [Google Scholar] [CrossRef]
- Wan, C.; Lu, Y.; Liu, T.; Zhao, L.; Yuan, W. Foaming of Low Density Polyethylene with Carbon Dioxide Based on Its in Situ Crystallization Behavior Characterized by High-Pressure Rheometer. Ind. Eng. Chem. Res. 2017, 56, 10702–10710. [Google Scholar] [CrossRef]
- Ma, W.; Andersson, A.; He, J.; Maurer, F.H.J. Free Volume Changes, Crystallization, and Crystal Transition Behavior of Syndiotactic Polystyrene in Supercritical CO2 Revealed by Positron Annihilation Lifetime Spectroscopy. Macromolecules 2008, 41, 5307–5312. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Zhai, J.; Zhang, L.; Lin, J.; Chen, F. Study on in situ visualization crystallization behavior and secondary nucleation model of polypropylene under supercritical N2. Compos. Sci. Technol. 2022, 230, 109770. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G. Poly(l-lactic acid) Crystallization in Pressurized CO2: An In Situ Microscopic Study and a New Model for Secondary Nucleation in Supercritical CO2. J. Phys. Chem. C 2020, 124, 9021–9034. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.; Zhai, J.; Zhang, L.; Chen, F.; Lin, J. Crystallization behavior and secondary nucleation model of PP/GN nanocomposites under supercritical N2. Compos. Commun. 2022, 36, 101362. [Google Scholar] [CrossRef]
- Lu, J.; Sui, X.; Yang, B.; Chen, J.; Cai, L.; Zhou, S.; Li, W.; Jiang, M.; Hao, S. Ultrafast in-situ transformation of graphite into graphene nanosheets by high current pulsed electron beam direct irradiation. Appl. Surf. Sci. 2021, 572, 151498. [Google Scholar] [CrossRef]
- Nagai, M.; Isoe, R.; Ishiguro, K.; Tominaga, H.; Shimizu, M. Graphite and graphene oxides catalyze bromination or alkylation in reaction of phenol with t -butylbromide. Chem. Eng. J. 2012, 207–208, 938–942. [Google Scholar] [CrossRef]
- Gai, Y.; Wang, W.; Xiao, D.; Tan, H.; Lin, M.; Zhao, Y. Exfoliation of Graphite into Graphene by a Rotor–Stator in Supercritical CO2: Experiment and Simulation. Ind. Eng. Chem. Res. 2018, 57, 8220–8229. [Google Scholar] [CrossRef]
- Navik, R.; Gai, Y.; Wang, W.; Zhao, Y. Curcumin-assisted ultrasound exfoliation of graphite to graphene in ethanol. Ultrason. Sonochem. 2018, 48, 96–102. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Pan, D.; Hu, S. Vertically Aligned and Interconnected Graphite and Graphene Oxide Networks Leading to Enhanced Thermal Conductivity of Polymer Composites. Polymers 2020, 12, 1121. [Google Scholar] [CrossRef]
- Nakamura, K.; Shimizu, S.; Umemoto, S.; Thierry, A.; Lotz, B.; Okui, N. Temperature Dependence of Crystal Growth Rate for α and β Forms of Isotactic Polypropylene. Polym. J. 2008, 40, 915–922. [Google Scholar] [CrossRef]
- Seeger, A.; Freitag, D.; Freidel, F.; Luft, G. Melting point of polymers under high pressure: Part I: Influence of the polymer properties. Thermochim. Acta 2004, 424, 175–181. [Google Scholar] [CrossRef]
- Xu, J.; Srinivas, S.; Marand, H.; Agarwal, P. Equilibrium Melting Temperature and Undercooling Dependence of the Spherulitic Growth Rate of Isotactic Polypropylene. Macromolecules 1998, 31, 8230–8242. [Google Scholar] [CrossRef]
- Mezghani, K.; Campbell, R.A.; Phillips, P.J. Lamellar Thickening and the Equilibrium Melting Point of Polypropylene. Macromolecules 1994, 27, 997–1002. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Lu, Y.; Wang, B.; Shen, C.; Chen, J.; Zhang, B. Later Stage Melting of Isotactic Polypropylene. Macromolecules 2020, 53, 2136–2144. [Google Scholar] [CrossRef]
- Supaphola, P.; Lin, J.-S. Crystalline memory effect in isothermal crystallization of syndiotactic. Polymer 2001, 42, 9617–9626. [Google Scholar] [CrossRef]









| Temperature (°C) | Pressure (MPa) | ||||
|---|---|---|---|---|---|
| 130 | 6 | 57.89 | 33.6 | 8.48 | 0.03 |
| 15 | 57.03 | 34.13 | 8.81 | 0.03 | |
| 23 | 57.7 | 32.9 | 9.37 | 0.03 | |
| 140 | 6 | 53.38 | 40.48 | 6.11 | 0.03 |
| 15 | 52.87 | 40.74 | 6.36 | 0.03 | |
| 23 | 54.06 | 39.1 | 6.81 | 0.03 |
| Temperature (°C) | Pressure (MPa) | ||||
|---|---|---|---|---|---|
| 130 | 6 | 53.44 | 26.82 | 19.52 | 0.22 |
| 15 | 52.23 | 26.23 | 21.16 | 0.38 | |
| 23 | 51.71 | 25.99 | 21.8 | 0.5 | |
| 140 | 6 | 53.53 | 27.73 | 18.58 | 0.16 |
| 15 | 52.31 | 27.12 | 20.3 | 0.27 | |
| 23 | 51.79 | 26.88 | 20.97 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Guan, Y.; Zhai, J.; Zhang, L.; Chen, F.; Lin, J. Refined Spherulites of PP Induced by Supercritical N2 and Graphite Nanosheet and Foaming Performance. Polymers 2023, 15, 1204. https://doi.org/10.3390/polym15051204
Liu Y, Guan Y, Zhai J, Zhang L, Chen F, Lin J. Refined Spherulites of PP Induced by Supercritical N2 and Graphite Nanosheet and Foaming Performance. Polymers. 2023; 15(5):1204. https://doi.org/10.3390/polym15051204
Chicago/Turabian StyleLiu, Ya, Yanjin Guan, Jiqiang Zhai, Lei Zhang, Fengjiao Chen, and Jun Lin. 2023. "Refined Spherulites of PP Induced by Supercritical N2 and Graphite Nanosheet and Foaming Performance" Polymers 15, no. 5: 1204. https://doi.org/10.3390/polym15051204