Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
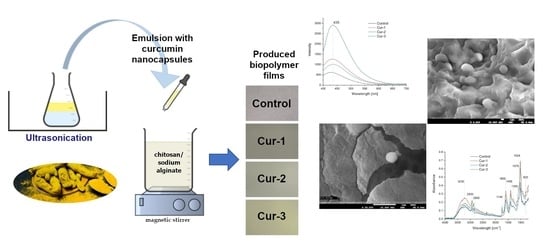
2.2. Films Preparation
2.2.1. Method for Obtaining Turmeric Extract
2.2.2. Preparation of Emulsion with Curcumin Nanocapsules
2.2.3. Preparation of the Chitosan–Alginate Matrix
2.2.4. Preparation of the Films
2.3. Films Characterization
2.3.1. FTIR Spectroscopy
2.3.2. UV-VIS Spectroscopy
2.3.3. Photoluminescence Spectroscopy
2.3.4. Determination of Water Content, Solubility and Degree of Swelling
2.3.5. Mechanical Tests
2.3.6. Film Color Measurement
2.3.7. Opacity of Films
2.3.8. Scanning Electron Microscopy
2.3.9. Determination of the Wetting Angles
2.3.10. Surface Free Energy (SFE)
2.3.11. Particle/Aggregate Size (DLS) and Zeta Potential
2.3.12. Antimicrobial Activity of the Films (Disc Diffusion Test)
2.3.13. Evaluation of the Susceptibility of the Tested Films to Soil Microorganisms Degradation
2.3.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dera, M.W.; Teseme, W.B. Mulugeta Wegari Dera, Wondemu Bogale Teseme. Review on the Application of Food Nanotechnology in Food Processing. Am. J. Eng. Technol. Manag. 2020, 5, 41–47. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in nanotechnology for food science and industry. Food Front. 2022, 3, 56–82. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Grzebieniarz, W.; Nowak, N.; Khachatryan, G.; Krzan, M.; Krystyjan, M.; Kosiński, J.; Khachatryan, K. The Preparation and Characterization of Quantum Dots in Polysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials 2021, 14, 7732. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Khachatryan, K.; Khachatryan, L.; Krzan, M.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Koronowicz, A.; Drozdowska, M.; Khachatryan, G. Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials 2021, 14, 3377. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Krzan, M.; Ciesielski, W.; Żarska, S.; Szczepankowska, J. Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers 2022, 14, 948. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Gohar, K.; Khachatryan, L.; Krystyjan, M.; Lenart-Boró, N.A.; Krzan, M.; Kulik, K.; Białecka, A.; Grabacka, M.; Nowak, N.; Khachatryan, K. Preparation of Nano/Microcapsules of Ozonated Olive Oil in Hyaluronan Matrix and Analysis of Physicochemical and Microbiological (Biological) Properties of the Obtained Biocomposite. Int. J. Mol. Sci. 2022, 23, 14005. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- Liu, T.; Li, J.; Tang, Q.; Qiu, P.; Gou, D.; Zhao, J. Chitosan-Based Materials: An Overview of Potential Applications in Food Packaging. Foods 2022, 11, 1490. [Google Scholar] [CrossRef]
- Ibañez-Peinado, D.; Ubeda-Manzanaro, M.; Martínez, A.; Rodrigo, D. Antimicrobial effect of insect chitosan on Salmonella Typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes survival. PLoS One 2020, 15, e0244153. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Júnior, L.M.; Fozzatti, C.R.; Jamróz, E.; Vieira, R.P.; Alves, R.M.V. Biopolymer-Based Films from Sodium Alginate and Citrus Pectin Reinforced with SiO2. Materials 2022, 15, 3881. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Grzesiakowska, A.; Kuchta-Gładysz, M.; Kawecka, A.; Grzebieniarz, W.; Nowak, N. The Functional and Application Possibilities of Starch/Chitosan Polymer Composites Modified by Graphene Oxide. Int. J. Mol. Sci. 2022, 23, 5956. [Google Scholar] [CrossRef]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary Curcumin Significantly Improves Obesity-Associated Inflammation and Diabetes in Mouse Models of Diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- Guo, J.; Cao, X.; Hu, X.; Li, S.; Wang, J. The anti-apoptotic, antioxidant and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol. Toxicol. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oghenejobo, M.; Opajobi, O.A.; Bethel, U.S.O.; Uzuegbu, U.E. Determination of Antibacterial Evaluation, Phytochemical Screening and Ascorbic Acid Assay of Turmeric (Curcuma longa). In Challenges and Advances in Pharmaceutical Research Volume 1; B P International: Hooghly, India, 2022; pp. 146–161. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Tosati, J.V.; de Oliveira, E.F.; Oliveira, J.V.; Nitin, N.; Monteiro, A.R. Light-activated antimicrobial activity of turmeric residue edible coatings against cross-contamination of Listeria innocua on sausages. Food Control 2018, 84, 177–185. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Curcumin and its uses in active and smart food packaging applications—A comprehensive review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Konieczna-Molenda, A.; Krzan, M.; Khachatryan, K. Preparation of nano/microcapsules of ozonated olive oil in chitosan matrix and analysis of physicochemical and microbiological properties of the obtained films. Innov. Food Sci. Emerg. Technol. 2022, 82, 103181. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Ciesielski, W.; Buksa, K.; Sikora, M. Preparation and characteristics of mechanical and functional properties of starch/Plantago psyllium seeds mucilage films. Starch/Staerke 2017, 69, 1700014. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Mello, J.M.M.; Dalcanton, F.; Macuvele, D.L.P.; Padoin, N.; Fiori, M.A.; Soares, C.; Riella, H.G. Mechanical, Thermal and Antimicrobial Properties of Chitosan-Based-Nanocomposite with Potential Applications for Food Packaging. J. Polym. Environ. 2020, 28, 1216–1236. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2019, 41, 690–698. [Google Scholar] [CrossRef]
- Chen, C.W.; Xie, J.; Yang, F.X.; Zhang, H.L.; Xu, Z.W.; Liu, J.L.; Chen, Y.J. Development of moisture-absorbing and antioxidant active packaging film based on poly(vinyl alcohol) incorporated with green tea extract and its effect on the quality of dried eel. J. Food Process. Preserv. 2018, 42, e13374. [Google Scholar] [CrossRef]
- Angeline, E.; Susidarti, R.A.; Rohman, A. Rapid authentication of turmeric powder adulterated with Curcuma zedoaria and Curcuma xanthorrhiza using FTIR-ATR spectroscopy and chemometrics. Int. J. Appl. Pharm. 2019, 11, 216–221. [Google Scholar] [CrossRef]
- Rohaeti, E.; Rafi, M.; Syafitri, U.D.; Heryanto, R. Fourier transform infrared spectroscopy combined with chemometrics for discrimination of Curcuma longa, Curcuma xanthorrhiza and Zingiber cassumunar. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1244–1249. [Google Scholar] [CrossRef]
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Quantification and classification of corn and sunflower oils as adulterants in olive oil using chemometrics and FTIR spectra. Sci. World J. 2012, 2012, 250795. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Studies on the Detection of Oleuropein from Extra Virgin Olive Oils Using Enzymatic Biosensors. Int. J. Mol. Sci. 2022, 23, 12569. [Google Scholar] [CrossRef]
- Gajic, I.M.S.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Omrani, Z.; Dadkhah Tehrani, A. New cyclodextrin-based supramolecular nanocapsule for codelivery of curcumin and gallic acid. Polym. Bull. 2020, 77, 2003–2019. [Google Scholar] [CrossRef]
- Bechnak, L.; El Kurdi, R.; Patra, D. Fluorescence Sensing of Nucleic Acid by Curcumin Encapsulated Poly(Ethylene Oxide)-Block-Poly(Propylene Oxide)-Block-Poly(Ethylene Oxide) Based Nanocapsules. J. Fluoresc. 2020, 30, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.; Sadeghizadeh, M.; Najafi, F.; Arab, S.S.; Pourhosseini, P.S. Application of a novel pH-responsive gemini surfactant for delivery of curcumin molecules. Mater. Res. Express 2020, 7, 065403. [Google Scholar] [CrossRef]
- Zaki, S.A.; Abd-Elrahman, M.I.; Abu-Sehly, A.A. Optical and electrical properties of amorphous Sb2S3 thin films: Effect of the film thickness. J. Non. Cryst. Solids 2021, 552, 120318. [Google Scholar] [CrossRef]
- Tüzemen, E.Ş.; Eker, S.; Kavak, H.; Esen, R. Dependence of film thickness on the structural and optical properties of ZnO thin films. Appl. Surf. Sci. 2009, 255, 6195–6200. [Google Scholar] [CrossRef]
- Benramache, S.; Temam, H.B.; Arif, A.; Guettaf, A.; Belahssen, O. Correlation between the structural and optical properties of Co doped ZnO thin films prepared at different film thickness. Optik 2014, 125, 1816–1820. [Google Scholar] [CrossRef]
- Coutts, T.J.; Young, D.L.; Li, X. Characterization of transparent conducting oxides. MRS Bull. 2000, 25, 58–65. [Google Scholar] [CrossRef]
- Azucena, R.C.I.; Roberto, C.L.J.; Martin, Z.R.; Rafael, C.Z.; Leonardo, H.H.; Gabriela, T.P.; Araceli, C.R. Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics 2019, 8, 43. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Kurkumina-naturalny polifenol o wielu właściwościach-rozwiązania technologiczne wspomagające farmakoterapię. Farm. Pol. 2020, 76, 603–610. [Google Scholar] [CrossRef]









| Films | Ingredients | ||
|---|---|---|---|
| Chitosan–Alginate Matrix (g) | Demineralized Water (mL) | Emulsion Containing Curcumin Nanocapsules (g) | |
| Control | 500 | 15 | 0 |
| Cur-1 | 500 | 10 | 5 |
| Cur-2 | 500 | 5 | 10 |
| Cur-3 | 500 | 0 | 15 |
| Sample | Water Content [%] | Solubility [%] | Degree of Swelling [%] |
|---|---|---|---|
| Control | 17.06 ± 0.58 a * | 24.04 ± 1.34 a | 30.93 ± 1.33 a |
| Cur-1 | 15.25 ± 0.56 b | 13.67 ± 0.49 b | 18.68 ± 0.60 b |
| Cur-2 | 9.82 ± 0.64 c | 12.71 ± 0.74 b | 11.22 ± 0.91 c |
| Cur-3 | 11.71 ± 0.74 d | 13.19 ± 0.69 b | 12.57 ± 0.76 c |
| Sample | Thickness (mm) | TS (MPa) | EAB (%) |
|---|---|---|---|
| Control | 0.090 ± 0.012 c * | 29.63 ± 3.35 b | 34.50 ± 2.55 a |
| Cur-1 | 0.111 ± 0.023 b | 12.38 ± 6.77 c | 29.49 ± 12.56 b |
| Cur-2 | 0.129 ± 0.007 b | 29.56 ± 4.75 a | 29.07 ± 3.83 b |
| Cur-3 | 0.178 ± 0.038 a | 11.33 ± 4.40 d | 40.93 ± 4.36 a |
| Sample | L*(D65) | a*(D65) | b*(D65) | O (-) |
|---|---|---|---|---|
| Control | 96.71 ± 0.19 b * | −0.29 ± 0.05 b | 3.71 ± 0.24 d | 1.39 ± 0.06 d |
| Cur-1 | 97.16 ± 0.10 a | −1.07 ± 0.06 c | 8.32 ± 0.58 c | 7.09 ± 0.45 a |
| Cur-2 | 94.14 ± 0.13 d | 0.49 ± 0.04 a | 17.23 ± 0.21 a | 5.85 ± 0.18 b |
| Cur-3 | 95.65 ± 0.54 c | −1.21 ± 0.16 d | 11.02 ± 1.66 b | 3.12 ± 0.24 c |
| Sample | Wetting Angles [°] | SFE [mJ/m2] | |||
|---|---|---|---|---|---|
| Water | DIM | Dispersion | Polar | Total | |
| Control | 79.98 ± 9.49 | 53.1 ± 5.40 | 32.53 | 5.08 | 37.61 |
| Cur-2 | 67.94 ± 4.69 | 41.05 ± 5.15 | 39.08 | 8.55 | 47.63 |
| Sample | Zeta Potential [mV]. | Particle Size [nm] |
| Control | −41.3 | 8509 |
| Cur-2 | −37.8 | 5130 |
| Sample | E. coli | A. fumigatus | P. expansum |
|---|---|---|---|
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Cur-2 | 24.3 ± 1.2 | 21.3 ± 0.5 | 3.0 ± 1.1 |
| Positive control | 20.5 ± 1.5 | 22.3 ± 0.6 | 20.3 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanisławska, N.; Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Makarewicz, M.; Krzan, M. Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules. Polymers 2023, 15, 919. https://doi.org/10.3390/polym15040919
Stanisławska N, Khachatryan G, Khachatryan K, Krystyjan M, Makarewicz M, Krzan M. Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules. Polymers. 2023; 15(4):919. https://doi.org/10.3390/polym15040919
Chicago/Turabian StyleStanisławska, Natalia, Gohar Khachatryan, Karen Khachatryan, Magdalena Krystyjan, Małgorzata Makarewicz, and Marcel Krzan. 2023. "Formation and Investigation of Physicochemical and Microbiological Properties of Biocomposite Films Containing Turmeric Extract Nano/Microcapsules" Polymers 15, no. 4: 919. https://doi.org/10.3390/polym15040919