Hybrid Microcapsules for Encapsulation and Controlled Release of Rosemary Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
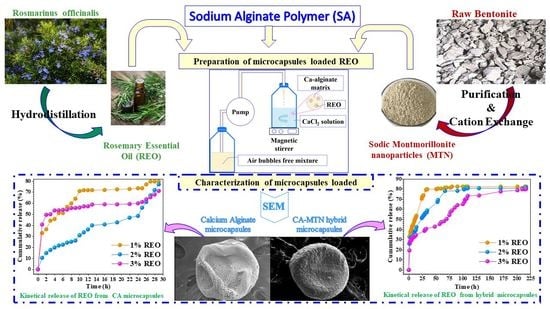
2.2. Preparation of Rosemary Essential Oil Loaded Microcapsules
2.3. Identification of Rosemary Essential Oil Chemotype
2.4. Characterization of Microcapsules
2.4.1. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR)
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Particle Size and Morphology
2.4.4. Loading Capacity and Encapsulation Efficiency
2.5. Determination of Antioxidant Activity
2.6. REO Release Kinetics
3. Results
3.1. Identification of Rosemary Essential Oil Chemotype
3.2. Characterization of Microcapsules
3.2.1. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR)
3.2.2. Thermogravimetric Analysis (TGA)
3.2.3. Particle Size and Morphology
3.2.4. Loading Capacity and Encapsulation Efficiency
3.3. Determination of the Antioxydant Activity
3.4. REO Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Silva Damasceno, E.T.; Almeida, R.R.; de Carvalho, S.Y.B.; de Carvalho, G.S.G.; Mano, V.; Pereira, A.C.; de Lima Guimarães, L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Ind. Crops Prod. 2018, 125, 85–94. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Mamusa, M.; Resta, C.; Sofroniou, C.; Baglioni, P. Encapsulation of volatile compounds in liquid media: Fragrances, flavors, and essential oils in commercial formulations. Adv. Colloid Interface Sci. 2021, 298, 102544. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2018, 17, 129–143. [Google Scholar] [CrossRef]
- Calderón-Santoyo, M.; Iñiguez-Moreno, M.; Barros-Castillo, J.C.; Miss-Zacarías, D.M.; Díaz, J.A.; Ragazzo-Sánchez, J.A. Microencapsulation of citral with Arabic gum and sodium alginate for the control of Fusarium pseudocircinatum in bananas. Iran. Polym. J. 2022, 31, 665–676. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. Adsorption of the methyl green dye pollutant from aqueous solution using mesoporous materials MCM-41 in a fixed-bed column. Heliyon 2020, 6, e03253. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Martinez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodriguez, S.A.; Roman, R.A.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Busatto, C.A.; Taverna, M.E.; Lescano, M.R.; Zalazar, C.; Estenoz, D.A. Preparation and Characterization of Lignin Microparticles-in-Alginate Beads for Atrazine Controlled Release. J. Polym. Environ. 2019, 27, 2831–2841. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Gasti, T.; Masti, S.P.; Malabadi, R.B.; Chougale, R.B. Polysaccharide-based blend films as a promising material for food packaging applications: Physicochemical properties. Iran. Polym. J. 2022, 31, 503–518. [Google Scholar] [CrossRef]
- Tondervik, A.; Klinkenberg, G.; Aachmann, F.L.; Svanem, B.I.; Ertesvag, H.; Ellingsen, T.E.; Valla, S.; Skjak-Braek, G.; Sletta, H. Mannuronan C-5 epimerases suited for tailoring of specific alginate structures obtained by high-throughput screening of an epimerase mutant library. Biomacromolecules 2013, 14, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Manso, S.; Cacho-Nerin, F.; Becerril, R.; Nerín, C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control 2013, 30, 370–378. [Google Scholar] [CrossRef]
- Tran, P.H.; Tran, T.T.; Park, J.B.; Lee, B.J. Controlled release systems containing solid dispersions: Strategies and mechanisms. Pharm. Res. 2011, 28, 2353–2378. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Luis, A.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Gupta, D.; Kothiyal, N.C.; Sharma, G.; Eldesoky, G.E.; Naushad, M. Preparation of a novel chitosan-g-poly(acrylamide)/Zn nanocomposite hydrogel and its applications for controlled drug delivery of ofloxacin. Int. J. Biol. Macromol. 2016, 84, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- El Miz, M.; Akichoh, H.; Berraaouan, D.; Salhi, S.; Tahani, A. Chemical and Physical Characterization of Moroccan Bentonite Taken from Nador (North of Morocco). Am. J. Chem. 2017, 7, 105–112. [Google Scholar] [CrossRef]
- Ribeiro, R.; Fernandes, L.; Costa, R.; Cavaleiro, C.; Salgueiro, L.; Henriques, M.; Rodrigues, M.E. Comparing the effect of Thymus spp. essential oils on Candida auris. Ind. Crops Prod. 2022, 178, 114667. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising vehicle for bioactive drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical Composition and Antioxidant Activity of the Essential Oils of Citral-Rich Chemotype Cinnamomum camphora and Cinnamomum bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Reboucas, L.M.; Sousa, A.C.C.; Sampaio, C.G.; Silva, L.M.R.; Costa, P.M.S.; Pessoa, C.; Brasil, N.; Ricardo, N. Microcapsules based on alginate and guar gum for co-delivery of hydrophobic antitumor bioactives. Carbohydr. Polym. 2023, 301, 120310. [Google Scholar] [CrossRef]
- Essifi, K.; Brahmi, M.; Berraaouan, D.; Ed-Daoui, A.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A.; Fernandez-Sanchez, J.F. Influence of Sodium Alginate Concentration on Microcapsules Properties Foreseeing the Protection and Controlled Release of Bioactive Substances. J. Chem. 2021, 2021, 5531479. [Google Scholar] [CrossRef]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of rosemary essential oil. LWT—Food Sci. Technol. 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Yeddes, W.; Djebali, K.; Aidi Wannes, W.; Horchani-Naifer, K.; Hammami, M.; Younes, I.; Saidani Tounsi, M. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: Optimized formulation using mixture design and response surface methodology. Int. J. Biol. Macromol. 2020, 154, 92–103. [Google Scholar] [CrossRef]
- Volic, M.; Pajic-Lijakovic, I.; Djordjevic, V.; Knezevic-Jugovic, Z.; Pecinar, I.; Stevanovic-Dajic, Z.; Veljovic, D.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS PharmSciTech 2005, 6, E209–E222. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Z.; Tu, L.; Han, Y.; Zhang, G.; Li, C. Encapsulation and characterization of slow-release microbial fertilizer from the composites of bentonite and alginate. Appl. Clay Sci. 2015, 109–110, 68–75. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.M.; Cooper, P.R.; Lawson, M.; Triffitt, J.T.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Zhou, G.; Xing, Z.; Tian, Y.; Jiang, B.; Ren, B.; Dong, X.; Yi, L. An environmental-friendly oil-based dust suppression microcapsules: Structure with chitosan derivative as capsule wall. Process Saf. Environ. Prot. 2022, 165, 453–462. [Google Scholar] [CrossRef]
- Chang, J.-H.; An, Y.U.; Cho, D.; Giannelis, E.P. Poly(lactic acid) nanocomposites: Comparison of their properties with montmorillonite and synthetic mica (II). Polymer 2003, 44, 3715–3720. [Google Scholar] [CrossRef]
- Alfatama, M.; Lim, L.Y.; Wong, T.W. Alginate-C18 Conjugate Nanoparticles Loaded in Tripolyphosphate-Cross-Linked Chitosan-Oleic Acid Conjugate-Coated Calcium Alginate Beads as Oral Insulin Carrier. Mol. Pharm. 2018, 15, 3369–3382. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos-Díaz, C.; Morales, E.; Rubilar, M.; Acevedo, F. Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocoll. 2017, 63, 139–148. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Novel Chitosan-Gelatin microcapsules containing rosemary essential oil for the preparation of bioactive and protective linen. Ind. Crops Prod. 2022, 178, 114549. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Silva Pereira, B.C.; Lopes, G.K.; Tristão Andrade, C. Encapsulation and antioxidant activity of assai pulp oil (Euterpe oleracea) in chitosan/alginate polyelectrolyte complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Faisant, N.; Siepmann, J.; Richard, J.; Benoit, J.P. Mathematical modeling of drug release from bioerodible microparticles: Effect of gamma-irradiation. Eur. J. Pharm. Biopharm. 2003, 56, 271–279. [Google Scholar] [CrossRef] [PubMed]







| Clay | CECEDA-Cu (meq/100 g) | BET (m2/g) | Pore Volume (cm3/g) | Zeta Potential (mV) |
|---|---|---|---|---|
| Raw | 68.74 | - | 0.009 | −27.77 |
| Homosodic | 91.66 | 94.25 | 0.28 | −24.72 |
| Name of the Compound | RT | Name of the Compound | RT |
|---|---|---|---|
| α-Pinene | 4.983 | Camphor | 8.308 |
| Camphene | 5.225 | Borneol | 8.625 |
| β-Pinene | 5.658 | 4-Terpineol | 8.775 |
| β-Myrcene | 5.817 | α-Terpieol | 8.967 |
| α-Terpinen | 6.258 | Isobornyl acetate | 10.325 |
| m-Cymene | 6.383 | Copaene | 11.600 |
| D-Limonene | 6.458 | Caryophyllene | 12.217 |
| 1.8-Cineol | 6.525 | α-Humulene | 12.650 |
| γ-Terpinen | 6.917 | Isoledene | 12.900 |
| cis-β-Terpineol | 7.067 | γ-Murolene | 13.383 |
| Terpinolene | 7.383 | δ-Cadinene, (+) | 13.475 |
| Linalool | 7.517 | Caryophyllene oxide | 14.283 |
| Microcapsule | CA Microcapsules | CA-MTN Hybrid Microcapsules |
|---|---|---|
| Diameter (mean ± SD) (mm) | 1.73 ± 0.01 | 1.64 ± 0.05 |
| Circularity (mean ± SD) | 0.92 ± 0.01 | 0.94 ± 0.01 |
| Aspect Ratio (mean ± SD) | 1.05 ± 0.01 | 1.17 ± 0.06 |
| Microcapsule | CA Microcapsules | CA-MTN Hybrid Microcapsules | ||||
|---|---|---|---|---|---|---|
| REO Concentration | 1% | 2% | 3% | 1% | 2% | 3% |
| LC (%) | 26.62 ± 0.19 | 49.93 ± 0.03 | 71.36 ± 0.1 | 28.58 ± 0.21 | 54.83 ± 0.12 | 73.6 ± 0.14 |
| EE (%) | 92.73 ± 0.14 | 81.946 ± 0.04 | 81.9 ± 0.04 | 83.58 ± 0.23 | 94.59 ± 0.12 | 83.59 ± 0.13 |
| Kinetic Parameters | CA-REO Microcapsules | CA-MTN-REO Hybrid Microcapsules |
|---|---|---|
| n | 0.29 | 0.28 |
| k | 0.18 | 0.12 |
| R2 | 0.93 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berraaouan, D.; Essifi, K.; Addi, M.; Hano, C.; Fauconnier, M.-L.; Tahani, A. Hybrid Microcapsules for Encapsulation and Controlled Release of Rosemary Essential Oil. Polymers 2023, 15, 823. https://doi.org/10.3390/polym15040823
Berraaouan D, Essifi K, Addi M, Hano C, Fauconnier M-L, Tahani A. Hybrid Microcapsules for Encapsulation and Controlled Release of Rosemary Essential Oil. Polymers. 2023; 15(4):823. https://doi.org/10.3390/polym15040823
Chicago/Turabian StyleBerraaouan, Doha, Kamal Essifi, Mohamed Addi, Christophe Hano, Marie-Laure Fauconnier, and Abdesselam Tahani. 2023. "Hybrid Microcapsules for Encapsulation and Controlled Release of Rosemary Essential Oil" Polymers 15, no. 4: 823. https://doi.org/10.3390/polym15040823