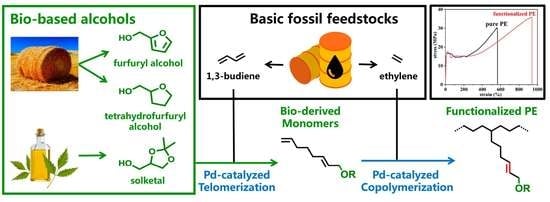
Polar-Functionalized Polyethylenes Enabled by Palladium-Catalyzed Copolymerization of Ethylene and Butadiene/Bio-Based Alcohol-Derived Monomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis of Pd-3
2.3. Synthesis of 2,7-Octadienyl Ether Monomers
2.4. A General Procedure for Ethylene Copolymerization
3. Results and Discussion
3.1. Synthesis of 2,7-Octadienyl Ether Monomers Derived from Telomerization of 1,3-Butadiene and Bio-Based Alcohols
3.2. Synthesis of Bio-Derived Functionalized Polyethylenes
3.3. Analysis of Copolymer Microstructures
3.4. Mechanical Properties of Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Keyes, A.; Basbug Alhan, H.E.; Ordonez, E.; Ha, U.; Beezer, D.B.; Dau, H.; Liu, Y.-S.; Tsogtgerel, E.; Jones, G.R.; Harth, E. Olefins and vinyl polar monomers: Bridging the gap for next generation materials. Angew. Chem. Int. Ed. 2019, 58, 12370–12391. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zou, C.; Chen, C. Material properties of functional polyethylenes from transition-metal-catalyzed ethylene–polar monomer copolymerization. Macromolecules 2022, 55, 1910–1922. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α-diimine catalysts. Acc. Chem. Res 2018, 51, 1831–1839. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, G.; Hu, X.; Jian, Z. Recent advances in nickel mediated copolymerization of olefin with polar monomers. Coord. Chem. Rev. 2021, 435, 213802. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef] [Green Version]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral nickel catalysts for olefin homo- and copolymerization: Relationships between catalyst structures and catalytic properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef]
- Pasini, D.; Takeuchi, D. Cyclopolymerizations: Synthetic tools for the precision synthesis of macromolecular architectures. Chem. Rev. 2018, 118, 8983–9057. [Google Scholar] [CrossRef]
- Luckham, S.L.J.; Nozaki, K. Toward the copolymerization of propylene with polar comonomers. Acc. Chem. Res. 2021, 54, 344–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Hu, X.; Wang, C.; Jian, Z. Advances on controlled chain walking and suppression of chain transfer in catalytic olefin polymerization. ACS Catal. 2022, 12, 14304–14320. [Google Scholar] [CrossRef]
- Xia, J.; Han, Y.-F.; Kou, S.; Zhang, Y.; Jian, Z. Exploring steric effect of electron-donating group in palladium and nickel mediated ethylene polymerization and copolymerization with polar monomers. Eur. Polym. J. 2021, 160, 110781. [Google Scholar] [CrossRef]
- Zhang, Y.; Jian, Z. Comprehensive picture of functionalized vinyl monomers in chain-walking polymerization. Macromolecules 2020, 53, 8858–8866. [Google Scholar] [CrossRef]
- Wu, Z.; Hong, C.; Du, H.; Pang, W.; Chen, C. Influence of ligand backbone structure and connectivity on the properties of phosphine-sulfonate Pd(II)/Ni(II) catalysts. Polymers 2017, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Zhang, Y.; Hu, X.; Ma, X.; Cui, L.; Zhang, J.; Jian, Z. Sterically very bulky aliphatic/aromatic phosphine-sulfonate palladium catalysts for ethylene polymerization and copolymerization with polar monomers. Polym. Chem. 2019, 10, 546–554. [Google Scholar] [CrossRef]
- Wang, X.; Nozaki, K. Selective chain-end functionalization of polar polyethylenes: Orthogonal reactivity of carbene and polar vinyl monomers in their copolymerization with ethylene. J. Am. Chem. Soc. 2018, 140, 15635–15640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, C. Influence of polyethylene glycol unit on palladium- and nickel-catalyzed ethylene polymerization and copolymerization. Angew. Chem. Int. Ed. 2017, 56, 14672–14676. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Jordan, R.F. Olefin insertion into a Pd-F bond: Catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed. 2017, 56, 1820–1824. [Google Scholar] [CrossRef]
- Na, Y.; Dai, S.; Chen, C. Direct synthesis of polar-functionalized linear low-density polyethylene (LLDPE) and low-density polyethylene (LDPE). Macromolecules 2018, 51, 4040–4048. [Google Scholar] [CrossRef]
- Gaikwad, S.R.; Deshmukh, S.S.; Koshti, V.S.; Poddar, S.; Gonnade, R.G.; Rajamohanan, P.R.; Chikkali, S.H. Reactivity of difunctional polar monomers and ethylene copolymerization: A comprehensive account. Macromolecules 2017, 50, 5748–5758. [Google Scholar] [CrossRef]
- Jian, Z.; Baier, M.C.; Mecking, S. Suppression of chain transfer in catalytic acrylate polymerization via rapid and selective secondary insertion. J. Am. Chem. Soc. 2015, 137, 2836–2839. [Google Scholar] [CrossRef]
- Ota, Y.; Ito, S.; Kuroda, J.; Okumura, Y.; Nozaki, K. Quantification of the steric influence of alkylphosphine–sulfonate ligands on polymerization, leading to high-molecular-weight copolymers of ethylene and polar monomers. J. Am. Chem. Soc. 2014, 136, 11898–11901. [Google Scholar] [CrossRef]
- Zhou, G.; Cui, L.; Mu, H.; Jian, Z. Custom-made polar monomers utilized in nickel and palladium promoted olefin copolymerization. Polym. Chem. 2021, 12, 3878–3892. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, J.; Song, J.; Zhang, J.; Ni, X.; Jian, Z. Combination of ethylene, 1,3-butadiene, and carbon dioxide into ester-functionalized polyethylenes via palladium-catalyzed coupling and insertion polymerization. Macromolecules 2019, 52, 2504–2512. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, Y.; Nozaki, K. Accessing divergent main-chain-functionalized polyethylenes via copolymerization of ethylene with a CO2/butadiene-derived lactone. J. Am. Chem. Soc. 2021, 143, 17953–17957. [Google Scholar] [CrossRef]
- Wang, X.; Seidel, F.W.; Nozaki, K. Synthesis of polyethylene with in-chain α, β-unsaturated ketone and isolated ketone units: Pd-catalyzed ring-opening copolymerization of cyclopropenone with ethylene. Angew. Chem. Int. Ed. 2019, 58, 12955–12959. [Google Scholar] [CrossRef]
- Cui, L.; Chen, M.; Chen, C.; Liu, D.; Jian, Z. Systematic studies on (co)polymerization of polar styrene monomers with palladium catalysts. Macromolecules 2019, 52, 7197–7206. [Google Scholar] [CrossRef]
- Jian, Z.; Mecking, S. Insertion polymerization of divinyl formal. Macromolecules 2016, 49, 4395–4403. [Google Scholar] [CrossRef]
- Jian, Z.; Mecking, S. Insertion homo- and copolymerization of diallyl ether. Angew. Chem. Int. Ed. 2015, 54, 15845–15849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagnani, D.E.; Tami, J.L.; Copley, G.; Clemons, M.N.; Getzler, Y.D.Y.L.; McNeil, A.J. 100th anniversary of macromolecular science viewpoint: Redefining sustainable polymers. ACS Macro Lett. 2021, 10, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, D.K.; Hillmyer, M.A. 50th anniversary perspective: There is a great future in sustainable polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Na, Y.; Chen, C. Catechol-functionalized polyolefins. Angew. Chem. Int. Ed. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Parisi, L.R.; Scheibel, D.M.; Lin, S.; Bennett, E.M.; Lodge, J.M.; Miri, M.J. Eugenol as renewable comonomer compared to 4-penten-1-ol in ethylene copolymerization using a palladium aryl sulfonate catalyst. Polymer 2017, 114, 319–328. [Google Scholar] [CrossRef]
- Rajput, B.S.; Pawal, S.B.; Bodkhe, D.V.; Rao, I.N.; Sainath, A.V.S.; Chikkali, S.H. Renewing polyethylene: Insertion copolymerization of sugar derived hydrophilic monomers with ethylene. Eur. Polym. J. 2020, 134, 109775. [Google Scholar] [CrossRef]
- Du, C.; Zhong, L.; Gao, J.; Zhong, S.; Liao, H.; Gao, H.; Wu, Q. Living (co)polymerization of ethylene and bio-based furfuryl acrylate using dibenzobarrelene derived α-diimine palladium catalysts. Polym. Chem. 2019, 10, 2029–2038. [Google Scholar] [CrossRef]
- Jian, Z.; Falivene, L.; Boffa, G.; Sánchez, S.O.; Caporaso, L.; Grassi, A.; Mecking, S. Direct synthesis of telechelic polyethylene by selective insertion polymerization. Angew. Chem. Int. Ed. 2016, 55, 14378–14383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, S.; Li, S.; Xu, G.; Chen, C. Direct synthesis of polar functionalized polyethylene thermoplastic elastomer. Macromolecules 2020, 53, 2539–2546. [Google Scholar] [CrossRef]
- Behr, A.; Becker, M.; Beckmann, T.; Johnen, L.; Leschinski, J.; Reyer, S. Telomerization: Advances and applications of a versatile reaction. Angew. Chem. Int. Ed. 2009, 48, 3598–3614. [Google Scholar] [CrossRef]
- Clement, N.D.; Routaboul, L.; Grotevendt, A.; Jackstell, R.; Beller, M. Development of palladium–carbene catalysts for telomerization and dimerization of 1,3-dienes: From basic research to industrial applications. Chem. Eur. J. 2008, 14, 7408–7420. [Google Scholar] [CrossRef]
- Jackstell, R.; Harkal, S.; Jiao, H.; Spannenberg, A.; Borgmann, C.; Röttger, D.; Nierlich, F.; Elliot, M.; Niven, S.; Cavell, K.; et al. An industrially viable catalyst system for palladium-catalyzed telomerizations of 1,3-butadiene with alcohols. Chem. Eur. J. 2004, 10, 3891–3900. [Google Scholar] [CrossRef]
- Jackstell, R.; Andreu, G.M.; Frisch, A.; Selvakumar, K.; Zapf, A.; Klein, H.; Spannenberg, A.; Röttger, D.; Briel, O.; Karch, R.; et al. A highly efficient catalyst for the telomerization of 1,3-dienes with alcohols: First synthesis of a monocarbenepalladium(0)-olefin complex. Angew. Chem. Int. Ed. 2002, 41, 986–989. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. Direct and tandem routes for the copolymerization of ethylene with polar functionalized internal olefins. Angew. Chem. Int. Ed. 2020, 59, 1206–1210. [Google Scholar] [CrossRef]
- Smedberg, A.; Hjertberg, T.; Gustafsson, B. Crosslinking reactions in an unsaturated low density polyethylene. Polymer 1997, 38, 4127–4138. [Google Scholar] [CrossRef]
- Fernandes, M.; Kaminsky, W. Copolymerization of ethylene with 2,7-octadienyl methyl ether in the presence of metallocene and nickel diimine catalysts. Macromol. Chem. Phys. 2009, 210, 585–593. [Google Scholar] [CrossRef]
- Guironnet, D.; Roesle, P.; Rünzi, T.; Göttker-Schnetmann, I.; Mecking, S. Insertion polymerization of acrylate. J. Am. Chem. Soc. 2009, 131, 422–423. [Google Scholar] [CrossRef] [Green Version]
- Wucher, P.; Goldbach, V.; Mecking, S. Electronic influences in phosphinesulfonato palladium(II) polymerization catalysts. Organometallics 2013, 32, 4516–4522. [Google Scholar] [CrossRef]
- Wang, C.; Xia, J.; Zhang, Y.; Hu, X.; Jian, Z. Photodegradable polar functionalized polyethylenes. Natl. Sci. Rev. 2023, nwad039. [Google Scholar] [CrossRef]
- Vogelsang, D.; Vondran, J.; Vorholt, A.J. One-step palladium catalysed synthetic route to unsaturated pelargonic C9-amides directly from 1,3-butadiene. J. Catal. 2018, 365, 24–28. [Google Scholar] [CrossRef]
- Behr, A.; Beckmann, T.; Schwach, P. Multiphase telomerisation of butadiene with acetic acid and acetic anhydride. J. Organomet. Chem. 2008, 693, 3097–3102. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Lanzafame, P.; Centi, G.; Perathoner, S. Catalysis for biomass and CO2 use through solar energy: Opening new scenarios for a sustainable and low-carbon chemical production. Chem. Soc. Rev. 2014, 43, 7562–7580. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Thermally self-healing polymeric materials: The next step to recycling thermoset polymers? Macromolecules 2009, 42, 1906–1912. [Google Scholar] [CrossRef] [Green Version]
- Odenwald, L.; Wimmer, F.P.; Mast, N.K.; Schußmann, M.G.; Wilhelm, M.; Mecking, S. Molecularly defined polyolefin vitrimers from catalytic insertion polymerization. J. Am. Chem. Soc. 2022, 144, 13226–13233. [Google Scholar] [CrossRef] [PubMed]






 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Cat | Monomer | c(M) (mol L−1) | Yield (g) | act. b (104) | Xc (mol %) | Mwd (104) | Mw/Mn d | Brs c | Tme (°C) |
| 1 | Pd-1 | OC8-FUR | 0.1 | 2.14 | 5.35 | 0.2 | 2.20 | 1.86 | 5.6 | 124.3 |
| 2 | Pd-1 | OC8-FUR | 0.3 | 0.59 | 1.48 | 0.6 | 2.07 | 1.94 | 4.5 | 120.8 |
| 3 | Pd-1 | OC8-FUR | 0.5 | 0.67 | 1.68 | 1.0 | 1.60 | 2.21 | 6.0 | 115.1 |
| 4 | Pd-1 | OC8-THF | 0.5 | 0.29 | 0.73 | 1.3 | 1.45 | 2.16 | 7.9 | 117.8 |
| 5 | Pd-1 | OC8-SOL | 0.5 | 0.57 | 1.43 | 1.9 | 1.41 | 2.16 | −f | 116.5 |
| 6 | Pd-2 | OC8-FUR | 0.1 | 0.65 | 1.63 | 0.2 | 4.16 | 1.97 | 4.2 | 123.3 |
| 7 | Pd-2 | OC8-FUR | 0.3 | 0.21 | 0.53 | 1.4 | 4.40 | 1.89 | 3.3 | 116.0 |
| 8 | Pd-2 | OC8-FUR | 0.5 | 0.23 | 0.58 | 2.0 | 4.79 | 2.13 | 7.7 | 114.4 |
| 9 | Pd-2 | OC8-THF | 0.5 | 0.18 | 0.45 | 2.1 | 3.34 | 2.12 | 9.6 | 116.0 |
| 10 | Pd-2 | OC8-SOL | 0.5 | 0.38 | 0.95 | 2.9 | 6.10 | 2.16 | −f | 117.2 |
| 11 | Pd-3 | OC8-FUR | 0.1 | 0.79 | 1.98 | 0.3 | 7.26 | 2.18 | 4.4 | 123.9 |
| 12 | Pd-3 | OC8-FUR | 0.3 | 0.49 | 1.23 | 0.5 | 7.52 | 1.87 | 0.7 | 118.0 |
| 13 | Pd-3 | OC8-FUR | 0.5 | 0.18 | 0.45 | 1.1 | 3.66 | 2.13 | 9.1 | 115.4 |
| 14 | Pd-3 | OC8-THF | 0.5 | 0.28 | 0.70 | 1.4 | 6.05 | 1.94 | 4.4 | 119.1 |
| 15 | Pd-3 | OC8-SOL | 0.5 | 0.31 | 0.78 | 1.6 | 5.98 | 2.15 | −f | 118.1 |
| 16 g | Pd-3 | OC8-SOL | 0.3 | 0.99 | 1.24 | 0.6 | 9.42 | 1.68 | −f | 118.3 |
| 17 g | Pd-3 | OC8-FUR | 0.5 | 0.35 | 0.44 | 1.6 | 2.75 | 1.96 | 1.9 | 112.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, Y.; Wang, C.; Zhang, Y.; Jian, Z. Polar-Functionalized Polyethylenes Enabled by Palladium-Catalyzed Copolymerization of Ethylene and Butadiene/Bio-Based Alcohol-Derived Monomers. Polymers 2023, 15, 1044. https://doi.org/10.3390/polym15041044
Zong Y, Wang C, Zhang Y, Jian Z. Polar-Functionalized Polyethylenes Enabled by Palladium-Catalyzed Copolymerization of Ethylene and Butadiene/Bio-Based Alcohol-Derived Monomers. Polymers. 2023; 15(4):1044. https://doi.org/10.3390/polym15041044
Chicago/Turabian StyleZong, Yanlin, Chaoqun Wang, Yixin Zhang, and Zhongbao Jian. 2023. "Polar-Functionalized Polyethylenes Enabled by Palladium-Catalyzed Copolymerization of Ethylene and Butadiene/Bio-Based Alcohol-Derived Monomers" Polymers 15, no. 4: 1044. https://doi.org/10.3390/polym15041044