On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents
Abstract
:1. Introduction
2. Hair Structure and Chemical Composition
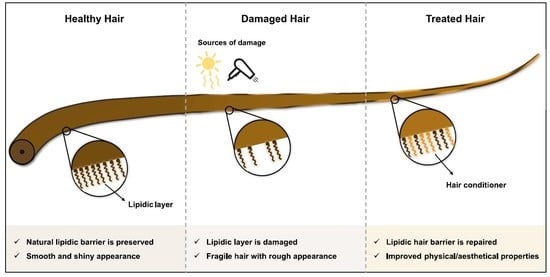
2.1. Hair Weathering
2.1.1. Mechanical Insults
2.1.2. Environmental Insults
2.1.3. Chemical Insults
2.1.4. Thermal Insults
3. Hair Care
4. Hair Conditioners
4.1. Classification of Hair Conditioners
- Neutralization of the negative charges of the hair fibers through the adsorption of cationic compounds onto the surface.
- Lubrication of the cuticles by restoring the hydrophobic character of the hair shaft.
- Restoring the lost proteins and enabling moisture retention through treatment with small proteins that penetrate the hair shaft.
4.2. Physicochemical Principles of the Hair-Conditioning Process
4.3. Alternative Conditioning Agents
4.4. Lignocellulosic Biomass as a Platform for Hair Care Products
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Lask, G.P.; Nelson, A. Comprehensive Aesthetic Rejuvenation: A Regional Approach; CRC Press: Boca Raton, FL, USA, 2011; Volume 8. [Google Scholar]
- Park, A.M.; Khan, S.; Rawnsley, J. Hair Biology: Growth and Pigmentation. Facial Plast. Surg. Clin. North Am. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Robbins, C.R. Interactions of Shampoo and Conditioner Ingredients with Hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–443. [Google Scholar]
- Robbins, C.R. Chemical Composition. In Chemical and Physical Behavior of Human Hair; Springer: New York, NY, USA, 1988; pp. 39–68. [Google Scholar]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. Introduction—Human Hair, Skin, and Hair Care Products. In Biophysics of Human Hair: Structural, Nanomechanical, and Nanotribological Studies; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–19. [Google Scholar]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo, A Review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Luengo, G.S.; Guzman, E.; Fernández-Peña, L.; Leonforte, F.; Ortega, F.; Rubio, R.G. Interaction of Polyelectrolytes and Surfactants on Hair Surfaces. Deposits and their Characterization. In Surface Science and Adhesion in Cosmetics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 401–449. [Google Scholar]
- Araújo, R.; Fernandes, M.; Cavaco-Paulo, A.; Gomes, A. Biology of Human Hair: Know Your Hair to Control It. In Biofunctionalization of Polymers and their Applications; Nyanhongo, G.S., Steiner, W., Gübitz, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 121–143. [Google Scholar]
- Hordinsky, M.; Caramori, A.P.A.; Donovan, J.C. Hair Physiology and Grooming. In Cosmetic Dermatology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 234–238. [Google Scholar]
- Morganti, P.; Morganti, G. Natural Polymers For Natural Hair: The Smart Use Of An Innovative Nanocarrier. In Nanocosmetics; Nanda, A., Nanda, S., Nguyen, T.A., Rajendran, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 267–285. [Google Scholar]
- McMichael, A.J.; Hordinsky, M.K. Hair and Scalp Diseases: Medical, Surgical, And Cosmetic Treatments; Informa Health Care: New York, NY, USA, 2008. [Google Scholar]
- Swift, J.A.; Smith, J.R. Microscopical investigations on the epicuticle of mammalian keratin fibres. J. Microsc. 2001, 204, 203–211. [Google Scholar] [CrossRef]
- Breakspear, S.; Smith, J.R.; Luengo, G. Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface. J. Struct. Biol. 2005, 149, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, S.; Tanamachi, H.; Ishikawa, K. Degradation of Hair Surface: Importance of 18-MEA and Epicuticle. Cosmetics 2019, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Marsh, J.; Gray, J.; Tosti, A. Understanding Hair Damage. In Healthy Hair; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–70. [Google Scholar]
- Menkart, J.; Mao, I.; Wolfram, L.J. Caucasian Hair, Negro Hair, and Wool: Similarities and Differences. J. Soc. Cosmet. Chem. 1966, 17, 769–787. [Google Scholar]
- Dekio, S.; Jidoi, J. Hair Low-sulfur Protein Composition does not Differ Electrophoretically among Different Races. J. Dermatol. 1988, 15, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Dekio, S.; Jidoi, J. Amounts of Fibrous Proteins and Matrix Substances in Hairs of Different Races. J. Dermatol. 1990, 17, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Franbourg, A.; Hallegot, P.; Baltenneck, F.; Toutain, C.; Leroy, F. Current research on ethnic hair. J. Am. Acad. Dermatol. 2003, 48, S115–S119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, I.P.; Bhushan, B. Effect of ethnicity and treatments on in situ tensile response and morphological changes of human hair characterized by atomic force microscopy. Acta Mater. 2008, 56, 3585–3597. [Google Scholar] [CrossRef]
- Takahashi, T. Unique Hair Properties that Emerge from Combinations of Multiple Races. Cosmetics 2019, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Bhushan, B.; Torgerson, P.M. Nanomechanical characterization of human hair using nanoindentation and SEM. Ultramicroscopy 2005, 105, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Osório, F.; Tosti, A. Hair weathering, Part 1: Hair structure and pathogenesis. Cosmet. Dermatol. 2011, 24, 533–538. [Google Scholar]
- Tosti, A.; Gray, J. Assessment of Hair and Scalp Disorders. J. Investig. Dermatol. Symp. Proc. 2007, 12, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos Nogueira, A.C.; Joekes, I. Hair color changes and protein damage caused by ultraviolet radiation. J. Photochem. Photobiol. B Biol. 2004, 74, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S. Photoaggravation of hair aging. Int. J. Trichology 2009, 1, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Hoting, E.; Zimmermann, M.; Hilterhaus-Bong, S. Photochemical alterations in human hair. I: Artificial irradiation and investigations of hair proteins. J. Soc. Cosmet. Chem. 1995, 46, 85–99. [Google Scholar]
- McMichael, A.J. Hair Breakage in Normal and Weathered Hair: Focus on the Black Patient. J. Investig. Dermatol. Symp. Proc. 2007, 12, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, Y.-D.; Hyun, H.-J.; Pi, L.-Q.; Jin, X.; Lee, W.-S. Hair shaft damage from heat and drying time of hair dryer. Ann. Dermatol. 2011, 23, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Yakawa, R.; Mamada, A.; Inoue, S.; Nagase, S.; Shibuichi, S.; Kariya, E.; Satoh, N. Influence of internal structures of hair fiber on hair appearance. III. Generation of light-scattering factors in hair cuticles and the influence on hair shine. J. Cosmet. Sci. 2003, 54, 353–366. [Google Scholar] [PubMed]
- The European Parliament; The Council of The European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union. Eur. Parliam. Counc. Eur. Union. 2009, L342, 59–209. [Google Scholar]
- Haskin, A.; Kwatra, S.G.; Aguh, C. Breaking the cycle of hair breakage: Pearls for the management of acquired trichorrhexis nodosa. J. Dermatol. Treat. 2017, 28, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.D. Healthy Hair: What Is it? J. Investig. Dermatol. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Trüeb, R.M. Aging of hair. J. Cosmet. Dermatol. 2005, 4, 60–72. [Google Scholar] [CrossRef]
- Bouillon, C. Shampoos. Clin. Dermatol. 1996, 14, 113–121. [Google Scholar] [CrossRef]
- Draelos, Z.D. Shampoos, conditioners, and camouflage techniques. Dermatol. Clin. 2013, 31, 173–178. [Google Scholar] [CrossRef]
- D’Souza, P.; Rathi, S.K. Shampoo and conditioners: What a dermatologist should know? Indian J. Dermatol. 2015, 60, 248–254. [Google Scholar] [CrossRef]
- Bolduc, C.; Shapiro, J. Hair care products: Waving, straightening, conditioning, and coloring. Clin. Dermatol. 2001, 19, 431–436. [Google Scholar] [CrossRef]
- Draelos, Z.D. Sunscreens and Hair Photoprotection. Dermatol. Clin. 2006, 24, 81–84. [Google Scholar] [CrossRef]
- Patil, A.; Ferritto, M.S. Polymers for Personal Care and Cosmetics: Overview. In Polymers for Personal Care and Cosmetics; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1148, pp. 3–11. [Google Scholar]
- Draelos, Z.K. Hair Cosmetics. Dermatol. Clin. 1991, 9, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.; Roberts, M.S.; Leite-Silva, V.R.; Walters, K. Cosmetic Formulation: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Yang, J. Hair Care Cosmetics. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–615. [Google Scholar]
- Gavazzoni Dias, M.F.R. Hair cosmetics: An overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Hair care: An Illustrated Dermatologic Handbook; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bhushan, B. Conditioner Thickness Distribution and Binding Interactions on Hair Surface. In Biophysics of Human Hair: Structural, Nanomechanical, and Nanotribological Studies; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–151. [Google Scholar]
- Robbins, C.; Reich, C.; Patel, A. Adsorption to keratin surfaces: A continuum between a charge-driven and a hydrophobically driven process. J. Soc. Cosmet. Chem. 1994, 45, 85–94. [Google Scholar]
- Cruz, C.F.; Costa, C.; Gomes, A.C.; Matamá, T.; Cavaco-Paulo, A. Human Hair and the Impact of Cosmetic Procedures: A Review on Cleansing and Shape-Modulating Cosmetics. Cosmetics 2016, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; LaTorre, C. Structural, Nanomechanical, and Nanotribological Characterization of Human Hair Using Atomic Force Microscopy and Nanoindentation. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1055–1170. [Google Scholar]
- Bujak, T.; Niziol-Lukaszewska, Z.; Ziemlewska, A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Biol. Macromol. 2020, 147, 973–979. [Google Scholar] [CrossRef]
- BIOWAYS. Increase public awareness of bio-based products and applications supporting the growth of the European bioeconomy (Grant agreement ID: 720762). 2016-2018. Available online: https://cordis.europa.eu/project/id/720762 (accessed on 10 January 2022).
- RoadToBio. Roadmap for the Chemical Industry in Europe towards a Bioeconomy (Grant agreement ID: 745623). 2017-2019. Available online: https://cordis.europa.eu/project/id/745623 (accessed on 10 January 2022).
- Delioglamnis, I.; Kouzi, E.; Tsagaraki, E.; Bougiouklis, M.; Tollias, I. Public Perception Of Bio-Based Products—Societal Needs And Concerns (Updated Version). BIOWAYS Deliverable D2.4; BIOWAYS (Grant agreement ID: 720762): 31/07/2018 2018. Available online: https://www.bioways.eu/download.php?f=307&l=en&key=f1d76fb7f2ae06b3ee3d4372a896d977 (accessed on 12 January 2022).
- Pfau, S.; Vos, J.; Dammer, L.; Arendt, O. Public Perception of Bio-based Products. RoadToBio Deliverable; D2.2; RoadToBio (Grant agreement ID: 745623): 2017. Available online: https://roadtobio.eu/uploads/publications/deliverables/RoadToBio_D22_Public_perception_of_bio-based_products.pdf (accessed on 12 January 2022).
- Karachaliou, E.; Tsagaraki, E.; Delioglamnis, I.; Kouzi, E. Public Perception Of Bio-Based Products. BIOWAYS Deliverable; D2.2; BIOWAYS (Grant agreement ID: 720762). 2017. Available online: https://www.bioways.eu/download.php?f=243&l=en&key=faf3e6f477c8183036b6eb591863b6e8 (accessed on 12 January 2022).
- European Commission. A renewed EU strategy 2011–14 for corporate social responsibility. Communication From the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Fortunati, S.; Martiniello, L.; Morea, D. The Strategic Role of the Corporate Social Responsibility and Circular Economy in the Cosmetic Industry. Sustainability 2020, 12, 5120. [Google Scholar] [CrossRef]
- Philippe, M.; Didillon, B.; Gilbert, L. Industrial commitment to green and sustainable chemistry: Using renewable materials & developing eco-friendly processes and ingredients in cosmetics. Green Chem. 2012, 14, 952–956. [Google Scholar] [CrossRef]
- Luengo, G.S.; Fameau, A.L.; Leonforte, F.; Greaves, A.J. Surface science of cosmetic substrates, cleansing actives and formulations. Adv. Colloid Interface Sci. 2021, 290. [Google Scholar] [CrossRef]
- Overkempe, C.; Annerling, A.; Van Ginkel, C.; Thomas, P.C.; Boltersdorf, D.; Speelman, J. Esterquats. In Novel surfactants: Preparation Applications And Biodegradability, Second Edition, Revised And Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 365–402. [Google Scholar]
- Azizova, M.; Archibald, E.A.; Tasker, R.; Chaudhuri, A. Hair treatment composition with naturally-derived peptide identical to human hair. United States Patent 9505820 B2, 2016. [Google Scholar]
- Tinoco, A.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022, 40, 591–605. [Google Scholar] [CrossRef]
- Rincón-Fontán, M.; Rodríguez-López, L.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Adsorption of natural surface active compounds obtained from corn on human hair. RSC Adv. 2016, 6, 63064–63070. [Google Scholar] [CrossRef] [Green Version]
- Ran, G.; Zhang, Y.; Song, Q.; Wang, Y.; Cao, D. The adsorption behavior of cationic surfactant onto human hair fibers. Colloids Surf. B. Biointerfaces 2009, 68, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Yorke, K.; Amin, S. High Performance Conditioning Shampoo with Hyaluronic Acid and Sustainable Surfactants. Cosmetics 2021, 8, 71. [Google Scholar] [CrossRef]
- Pérusse, D.; Guégan, J.P.; Rolland, H.; Guilbot, J.; Benvegnu, T. Efficient solvent-free cationization of alkylpolyglycoside based surfactant compositions using natural glycine betaine. Green Chem. 2016, 18, 1664–1673. [Google Scholar] [CrossRef]
- Hayes, D.G.; Smith, G.A. Biobased Surfactants: Overview and Industrial State of the Art. In Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; AOCS Press: Urbana, IL, USA, 2019; pp. 3–38. [Google Scholar]
- Stjerndahl, M.; Lundberg, D.; Holmberg, A.K. Cleavable Surfactants. In Novel surfactants:Preparation Applications And Biodegradability, Second Edition, Revised And Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 317–345. [Google Scholar]
- Stepan Company. STEPANQUAT® Helia-Product Bulletin. Available online: https://www.stepan.com/products-markets/product/STEPANQUATHelia.html (accessed on 10 January 2022).
- Stepan Company. STEPANQUAT® Soleil-Product Bulletin. Available online: https://www.stepan.com/products-markets/product/STEPANQUATSoleil.html (accessed on 15 January 2022).
- Ajayi, O.; Davies, A.; Amin, S. Impact of Processing Conditions on Rheology, Tribology and Wet Lubrication Performance of a Novel Amino Lipid Hair Conditioner. Cosmetics 2021, 8, 77. [Google Scholar] [CrossRef]
- Inolex Company. AminoSensyl™ HC. Available online: https://inolex.com/pc/Products/Amino-Lipid-Technology/AminoSensyl-HC (accessed on 16 January 2022).
- Uthayakumaran, S.; Wrigley, C.W. Wheat: Characteristics and quality requirements. In Cereal Grains: Assessing and Managing Quality; Wrigley, C.W., Batey, I.L., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 59–111. [Google Scholar]
- Wang, S.; Meng, D.; Wang, S.; Zhang, Z.; Yang, R.; Zhao, W. Modification of wheat gluten for improvement of binding capacity with keratin in hair. R. Soc. Open Sci. 2018, 5, 171216. [Google Scholar] [CrossRef] [Green Version]
- Savary, G.; Grisel, M.; Picard, C. Cosmetics and Personal Care Products. In Natural Polymers: Industry Techniques and Applications; Olatunji, O., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 219–261. [Google Scholar]
- Basu, P. Biomass Characteristics. In Biomass Gasification, Pyrolysis and Torrefaction (Third Edition); Basu, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–91. [Google Scholar]
- Mellou, F.; Varvaresou, A.; Papageorgiou, S. Renewable sources: Applications in personal care formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting lignin: A tour through its structural features, characterization methods and applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Patil, N.D.; Tanguy, N.R.; Yan, N. Lignin Interunit Linkages and Model Compounds. In Lignin in Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–47. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Xu, C. Synthesis of phenol–formaldehyde resol resins using organosolv pine lignins. Eur. Polym. J. 2009, 45, 3380–3388. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, M.D.M.; da Paz Leôncio Alves, S.; da Cruz Filho, I.J.; de Sousa, G.F.; de Souza Silva, G.A.; do Nascimento Santos, D.K.D.; do Carmo Alves de Lima, M.; de Moraes Rocha, G.J.; de Souza, I.A.; de Melo, C.M.L. Characterization of a lignin from Crataeva tapia leaves and potential applications in medicinal and cosmetic formulations. Int. J. Biol. Macromol. 2021, 180, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for white natural sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Gagliardini, A.; Lohani, A. From Cosmetics to Innovative Cosmeceuticals—Non-Woven Tissues as New Biodegradable Carriers. Cosmetics 2021, 8, 65. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.L.; Morganti, G.; Cardillo, M.; Chianese, A. Green Nanotechnology Serving the Bioeconomy: Natural Beauty Masks to Save the Environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R. Nano-Structured Lignin as Green Antioxidant and UV Shielding Ingredient for Sunscreen Applications. Antioxidants 2021, 10, 274. [Google Scholar] [CrossRef]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound Driven Assembly of Lignin into Microcapsules for Storage and Delivery of Hydrophobic Molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, M.P.; Ugartondo, V.; Mitjans, M. Potential applications of antioxidant lignins from different sources. Ind. Crops Prod. 2008, 27, 220–223. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Coltelli, M.-B. Smart and Sustainable Hair Products Based on Chitin-Derived Compounds. Cosmetics 2021, 8, 20. [Google Scholar] [CrossRef]
- Panariello, L.; Vannozzi, A.; Morganti, P.; Coltelli, M.-B.; Lazzeri, A. Biobased and Eco-Compatible Beauty Films Coated with Chitin Nanofibrils, Nanolignin and Vitamin E. Cosmetics 2021, 8, 27. [Google Scholar] [CrossRef]
- Morganti, P.; Coltelli, M.-B. A New Carrier for Advanced Cosmeceuticals. Cosmetics 2019, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic potential of lignin extracts from alkaline-treated sugarcane bagasse: Optimization of extraction conditions using response surface methodology. Int. J. Biol. Macromol. 2020, 153, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chemyunion. Allinea. Available online: https://chemyunion.com/en/hair-care/allinea (accessed on 10 February 2022).










| Classification | Application | Conditioning Agents Used |
|---|---|---|
| Shampoos “2 in 1” | Shampoo with dual function—cleaning and conditioning. Conditioning additives are incorporated to impart manageability, gloss, and antistatic properties to the hair. Recommended for dry, damaged, or chemically treated hair. | Hydrolyzed protein, silicones, glycerin, polyvinylpyrrolidone or quaternary conditioners. |
| Instant conditioners | Intended to be applied after shampooing and rinsed after a short period of time (around 5 min). Ideal for daily use in slightly damaged hair to reduce the effect of shampoo and improve daily manageability. | Quaternary conditioners, such as behentrimonium chloride and stearalkonium chloride. |
| Deep/intensive conditioners | More concentrated than instant conditioners and should be left on the hair for 20 to 30 min. Recommended for extremely dry hair or before chemical treatment, such as coloring and waving. | Higher amounts of quaternary conditioners in addition to proteins. |
| Leave-in conditioners | Designed to be applied after shampooing and conditioning, and not rinsed out. These products can be applied daily in wet or dry hair and are ideal for preventing damage from routine grooming. | Silicones, oils, polyvinylpyrrolidone or other film-forming agents. |
| Blow drying conditioners | They are applied to towel-dried hair before blow-drying and styling and may provide photoprotection and prevent heat damage. Useful for people with fine hair and excessive scalp sebum. | Same agents as instant conditioners but do not contain oil. |
| Hair thickeners | They coat the hair shaft, increasing their diameter and giving the illusion of thick hair. They usually contain proteins as conditioners and are also applied to towel-dried hair before styling. | Film-forming agents, such as silicones or hydrolyzed proteins, such as keratin K31. |
| Classification | Mode of Action | Ingredients |
|---|---|---|
| Cationic conditioners | Act by neutralization of the negative charges of damaged hair by deposition of positively charged molecules on the hair surface. The softness and smooth appearance of the hair are achieved by the reduction of static electricity of the cuticles. Excellent for chemically processed hair. | Quaternary ammonium compounds: cetrimonium chloride, stearalkonium chloride, etc. |
| Film-forming conditioners | Act by deposition of polymers that form a film that fills the defects in cuticles surface and coat the hair shaft, restoring its softness and shine. They can also be positively charged and reduce the static electricity of anionic damaged hair shaft. | Film-forming agents, such as polyvinylpyrrolidone (PVP), silicones and oils. |
| Protein-based conditioners | Contain amino acids and small polypeptide fragments of hydrolyzed proteins that can penetrate the hair shaft and repair the damaged hair by restoring the lost proteins and improve the hair’s strength. The excess proteins are rinsed out when washing the hair, so their effect is only temporary. | Many different protein sources: animal protein, eggs, placenta, collagen, keratin, beer, among others. |
| Name | Function | Examples of Derivatives Used in Cosmetics [INCI] |
|---|---|---|
| Starch (maize, potato, tapioca…) | Conditioner; softening agent; thickener | Aluminium Starch Octenyl Succinate Distarch Phosphate Hydroxypropyl Starch Phosphate Starch Hydroxypropyltrimonium Chloride. |
| Galactomannan gums (guar and locust bean) | Film former; stabilizer; thickener | Hydroxypropyl Guar Guar Hydroxypropyltrimonium Chloride Locust Bean Hydroxypropyltrimonium Chloride |
| Hydrolyzed wheat | Conditioner; film former; antistatic; tensor | Hydrolyzed Wheat Protein/Dimethicone PEG-7 Acetate Hydrolyzed Wheat Protein/PEG-20 Acetate Copolymer Hydrolyzed Wheat Protein PG-Propyl Silanetriol |
| Hydrolyzed keratin | Conditioner; moisturizer | Cocodimonium Hydroxypropyl Hydrolyzed Keratin Hydrolyzed Keratin PG-Propyl Methylsilanediol |
| Collagen, gelatin, and hydrolyzed collagen | Conditioner; film former; moisturizer; hydrating; | Cocodimonium Hydroxypropyl Hydrolyzed Collagen Laurdimonium Hydroxypropyl Hydrolyzed Collagen Isostearoyl Hydrolyzed Collagen |
| Chitosan, hydrolyzed chitosan and chitin | Conditioner; film former; thickener; chelating agent; hydrating | Chitosan Lactate Chitosan PCA |
| Hydrolyzed silk | Hair conditioner; antistatic; humectants | Hydrolyzed Silk PG-Propyl Methylsilanediol Sodium Lauroyl Hydrolyzed Silk |
| Cellulose derivatives | Film former; emulsion stabilizer; viscosity control | Cetyl Hydroxyethylcellulose Hydroxyethylcellulose Hydroxypropylcellulose |
| Cationic cellulose derivatives | Antistatic, film forming | Polyquaternium-10 (Cationic hydroxyethyl cellulose) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. https://doi.org/10.3390/polym15030608
Fernandes C, Medronho B, Alves L, Rasteiro MG. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers. 2023; 15(3):608. https://doi.org/10.3390/polym15030608
Chicago/Turabian StyleFernandes, Catarina, Bruno Medronho, Luís Alves, and Maria Graça Rasteiro. 2023. "On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents" Polymers 15, no. 3: 608. https://doi.org/10.3390/polym15030608