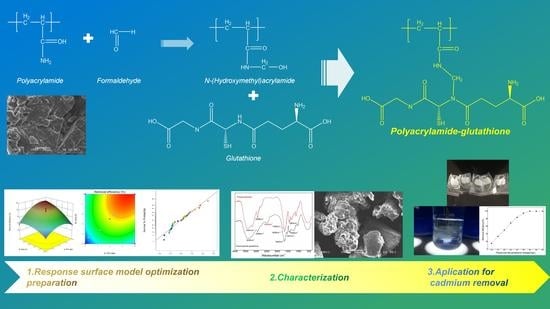
Preparation of Heavy Metal Trapping Flocculant Polyacrylamide–Glutathione and Its Application for Cadmium Removal from Water
Multifunctional Polymers Used in Agricultural Application and Environmental Treatment
)
Abstract
:1. Introduction
2. Experiment
2.1. Material
2.2. Preparation
2.2.1. Polyacrylamide Synthesis
2.2.2. Glutathione Grafting
2.3. Characterization
2.4. Flocculation Test
3. Discussion
3.1. Response Surface Method to Optimize the Preparation
3.1.1. Single-Factor Test for Synthesis Conditions
3.1.2. Experiments Design for Response Surface Optimization
3.1.3. ANOVA and Model Significance Analysis
3.1.4. Response Surface Model Analysis
3.1.5. Model Optimization Results Validation
3.2. Characterization
3.2.1. Functional Group Analysis via FTIR
3.2.2. Surface Morphological Analysis via SEM
3.2.3. Analysis of the flocculation mechanism
3.3. Performance Testing and Flocculation Mechanism
3.3.1. Cadmium Removal Performance Test
3.3.2. Analysis of the Flocculation Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for Coagulation/Flocculation Processes—An Eco-Friendly Approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/Flocculation in Dewatering of Sludge: A Review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Rostami Najafabadi, Z.; Soares, J.B.P. Water Soluble Polymer Flocculants: Synthesis, Characterization, and Performance Assessment. Macromol. Mater. Eng. 2018, 304, 1800526. [Google Scholar] [CrossRef] [Green Version]
- Siddeeg, S.M.; Tahoon, M.A.; Ben Rebah, F. Agro-Industrial Waste Materials and Wastewater as Growth Media for Microbial Bioflocculants Production: A Review. Mater. Res. Express 2019, 7, 012001. [Google Scholar] [CrossRef]
- Abbasi Moud, A. Polymer Based Flocculants: Review of Water Purification Applications. J. Water Process Eng. 2022, 48, 102938. [Google Scholar] [CrossRef]
- Wang, Y.; Pushiri, H.; Looi, L.J.; Zulkeflee, Z. Applications of Bioflocculants for Heavy Metals Removal: A Systematic Review. Int. J. Environ. Res. 2022, 16, 73. [Google Scholar] [CrossRef]
- O’Shea, J.-P.; Qiao, G.G.; Franks, G.V. Solid–Liquid Separations with a Temperature-Responsive Polymeric Flocculant: Effect of Temperature and Molecular Weight on Polymer Adsorption and Deposition. J. Colloid Interface Sci. 2010, 348, 9–23. [Google Scholar] [CrossRef] [PubMed]
- BOLTO, B.; GREGORY, J. Organic Polyelectrolytes in Water Treatment. Water Res. 2007, 41, 2301–2324. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Jia, S.; Yang, Z.; Yang, W.; Zhang, T.; Zhang, S.; Yang, X.; Dong, Y.; Wu, J.; Wang, Y. Removal of Cu(II) and Tetracycline Using an Aromatic Rings-Functionalized Chitosan-Based Flocculant: Enhanced Interaction between the Flocculant and the Antibiotic. Chem. Eng. J. 2016, 283, 495–503. [Google Scholar] [CrossRef]
- Kan, K.H.M.; Li, J.; Wijesekera, K.; Cranston, E.D. Polymer-Grafted Cellulose Nanocrystals as PH-Responsive Reversible Flocculants. Biomacromolecules 2013, 14, 3130–3139. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Evaluation of Structural Effects on the Flocculation Performance of a Co-Graft Starch-Based Flocculant. Water Res. 2017, 118, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liang, C.-Z.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Combination of Forward Osmosis (FO) Process with Coagulation/Flocculation (CF) for Potential Treatment of Textile Wastewater. Water Res. 2016, 91, 361–370. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, H.; Wang, Y.; Chen, X.; Zhao, C.; An, Y.; Tang, X. A Novel Carboxyl-Rich Chitosan-Based Polymer and Its Application for Clay Flocculation and Cationic Dye Removal. Sci. Total Environ. 2018, 640–641, 107–115. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; An, Y.; Ren, J.; Zheng, X.; Zhao, C.; Zhang, S. Ultrasound-Assisted Synthesis of the β-Cyclodextrin Based Cationic Polymeric Flocculants and Evaluation of Flocculation Performance: Role of β-Cyclodextrin. Sep. Purif. Technol. 2019, 228, 115735. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a Novel Dextran-Based Flocculant on Treatment of Dye Wastewater: Effect of Kaolin Particles. Sci. Total Environ. 2018, 640–641, 243–254. [Google Scholar] [CrossRef]
- Hu, P.; Xi, Z.; Li, Y.; Li, A.; Yang, H. Evaluation of the Structural Factors for the Flocculation Performance of a Co-Graft Cationic Starch-Based Flocculant. Chemosphere 2020, 240, 124866. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shah, K.J.; Sun, W.; Zheng, H. Performance Evaluation of Chitosan-Based Flocculants with Good PH Resistance and High Heavy Metals Removal Capacity. Sep. Purif. Technol. 2019, 215, 208–216. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of Carboxymethyl Cellulose and Polyacrylamide Graft Copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Gregory, J.; Barany, S. Adsorption and Flocculation by Polymers and Polymer Mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Baudouin-Cornu, P.; Lagniel, G.; Kumar, C.; Huang, M.-E.; Labarre, J. Glutathione Degradation Is a Key Determinant of Glutathione Homeostasis. J. Biol. Chem. 2012, 287, 4552–4561. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Nasim, T.; Patra, A.; Ghosh, S.; Panda, A.B. Microwave Assisted Synthesis of Polyacrylamide Grafted Dextrin (Dxt-g-PAM): Development and Application of a Novel Polymeric Flocculant. Int. J. Bio. Macro. 2010, 47, 623–631. [Google Scholar] [CrossRef]
- Adhikary, P.; Krishnamoorthi, S.; Singh, R.P. Synthesis and Characterization of Grafted Carboxymethyl Guar Gum. J. Appl. Polym. Sci. 2010, 120, 2621–2626. [Google Scholar] [CrossRef]
- Tajbakhsh, S.F.; Mohmmadipour, R.; Janani, H. One-Pot Production of a Graft Copolymer of Cationic Starch and Cationic Polyacrylamide Applicable as Flocculant for Wastewater Treatment. J. Macromol. Sci. Part A 2022, 59, 698–710. [Google Scholar] [CrossRef]
- Zheng, H.; Ma, J.; Zhai, J.; Zhu, C.; Tang, X.; Liao, Y.; Qian, L.; Sun, Y. Optimization of Flocculation Process by Response Surface Methodology for Diethyl Phthalate Removal Using Anionic Polyacrylamide. Desalination Water Treat. 2013, 52, 5390–5400. [Google Scholar] [CrossRef]
- Dawood, A.; Li, Y. Modeling and Optimization of New Flocculant Dosage and PH for Flocculation: Removal of Pollutants from Wastewater. Water 2013, 5, 342–355. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Vining, G.G.; Borror, C.M.; Kowalski, S.M. Response Surface Methodology: A Retrospective and Literature Survey. J. Qual. Technol. 2004, 36, 53–77. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, J. Cationic Polyacrylamide: Synthesis and Application in Sludge Dewatering Treatment. Asian J. Chem. 2014, 26, 629–633. [Google Scholar] [CrossRef]
- Zheng, H.; Ma, J.; Ji, F.; Tang, X.; Chen, W.; Zhu, J.; Liao, Y.; Tan, M. Synthesis and Application of Anionic Polyacrylamide in Water Treatment. Asian J. Chem. 2013, 25, 7071–7074. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, H.; Dai, L.; Li, F.; Zhu, G.; Guan, Q.; Sun, Y.; Tang, X. Hydrophobically Modified Polyacrylamide Synthesis and Application in Water Treatment. Asian J. Chem. 2014, 26, 5923–5927. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Zhang, Y.; Guo, J.; Sun, Y.; Liu, B.; Tang, X. Advances in the Initiation System and Synthesis Methods of Cationic Poly-Acrylamide: A Review. Mini-Rev. Org. Chem. 2016, 13, 109–117. [Google Scholar] [CrossRef]
- Ahmad, R.; Ansari, K.; Ejaz, M.O. Enhanced Sequestration of Heavy Metals from Aqueous Solution on Polyacrylamide Grafted with Cell@Fe3O4 Nanocomposite. Emergent Mater. 2022, 5, 1517–1531. [Google Scholar] [CrossRef]
- Gendensuren, B.; Sugartseren, N.; Kim, M.; Oh, E.-S. Incorporation of Aniline Tetramer into Alginate-Grafted-Polyacrylamide as Polymeric Binder for High-Capacity Silicon/Graphite Anodes. Chem. Eng. J. 2022, 433, 133553. [Google Scholar] [CrossRef]
- Wang, L.; Shen, M.; Hou, Q.; Wu, Z.; Xu, J.; Wang, L. 3D Printing of Reduced Glutathione Grafted Gelatine Methacrylate Hydrogel Scaffold Promotes Diabetic Bone Regeneration by Activating PI3K/Akt Signaling Pathway. Int. J. Biol. Macromol. 2022, 222, 1175–1191. [Google Scholar] [CrossRef]
- Kiran; Tiwari, R.; Krishnamoorthi, S.; Kumar, K. Synthesis of Cross-Linker Devoid Novel Hydrogels: Swelling Behaviour and Controlled Urea Release Studies. J. Environ. Chem. Eng. 2019, 7, 103162. [Google Scholar] [CrossRef]
- Varvarenko, S.; Voronov, A.; Samaryk, V.; Tarnavchyk, I.; Nosova, N.; Kohut, A.; Voronov, S. Covalent Grafting of Polyacrylamide-Based Hydrogels to a Polypropylene Surface Activated with Functional Polyperoxide. React. Funct. Polym. 2010, 70, 647–655. [Google Scholar] [CrossRef]
- Nazlabadi, E.; Alavi Moghaddam, M.R.; Karamati-Niaragh, E. Simultaneous Removal of Nitrate and Nitrite Using Electrocoagulation/Floatation (ECF): A New Multi-Response Optimization Approach. J. Eviron. Manag. 2019, 250, 109489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, B.; Li, S.; Lin, X.; Chen, M.; Xu, X. Synthesis and Properties of Rosin-Based Composite Acrylamide Hydrogels. J. Renew. Mater. 2023, 11, 853–865. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.; Yi, P.; Zhou, S.; Wang, Y.; Yang, D. Design, Preparation and Properties of New Polyacrylamide Based Composite Nano-Microspheres with like “Ball in Ball” Structure. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130037. [Google Scholar] [CrossRef]
- Cao, Q.-L.; Wang, R.-T.; Duan, J.-Y.; Dong, G.-Y. Two Stable Cadmium(II) Coordination Polymers for Fluorimetric Detection of Tetracycline and Fe3+ Ions. J. Solid State Chem. 2022, 307, 122816. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S.; Batool, S.; Yusuff, A.S. An Overview of the Use of Water-Stable Metal-Organic Frameworks in the Removal of Cadmium Ion. J. Environ. Chem. Eng. 2023, 11, 109131. [Google Scholar] [CrossRef]
- Kirby, B.J.; Hasselbrink, E.F. Zeta Potential of Microfluidic Substrates: 2. Data for Polymers. Electrophoresis 2004, 25, 203–213. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, N.; Guo, J.; Lu, L.; Li, N.; Hu, T.; Zhu, Z.; Gao, X.; Li, X.; Jiang, L.; et al. The Aggregation of Natural Inorganic Colloids in Aqueous Environment: A Review. Chemosphere 2023, 310, 136805. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Yu, L.; Li, K.; Zhang, Y.; Wang, J.; Wang, J. Tuneable Ion Transport by Electrically Responsive Membranes under Electrical Assistance. J. Membr. Sci. 2022, 663, 121046. [Google Scholar] [CrossRef]











| Code | Polyacrylamide/Glutathione Mass Ratio | Reaction Time (h) | Formaldehyde Dosage (mL/L) | pH |
|---|---|---|---|---|
| −1 | 1.35 | 30 | 0.33 | 0.5 |
| 0 | 4.05 | 32.5 | 0.665 | 2.25 |
| 1 | 6.75 | 45 | 1 | 4 |
| Polyacrylamide/Glutathione Mass Ratio | Reaction Time (h) | Formaldehyde Dosage (mL/L) | pH | Extraction Rate of Cadmium (%) | |
|---|---|---|---|---|---|
| 1 | 3 | 0.5 | 5 | 1 | 82.16 |
| 2 | 15 | 0.5 | 5 | 1 | 84.36 |
| 3 | 3 | 4 | 5 | 1 | 89.55 |
| 4 | 15 | 4 | 5 | 1 | 96.31 |
| 5 | 9 | 2.25 | 3 | 0.4 | 84.26 |
| 6 | 9 | 2.25 | 7 | 0.4 | 91.02 |
| 7 | 9 | 2.25 | 3 | 1.6 | 93.5 |
| 8 | 9 | 2.25 | 7 | 1.6 | 96.55 |
| 9 | 3 | 2.25 | 5 | 0.4 | 82.21 |
| 10 | 15 | 2.25 | 5 | 0.4 | 91.57 |
| 11 | 3 | 2.25 | 5 | 1.6 | 87.62 |
| 12 | 15 | 2.25 | 5 | 1.6 | 98.07 |
| 13 | 9 | 0.5 | 3 | 1 | 92.58 |
| 14 | 9 | 4 | 3 | 1 | 92.33 |
| 15 | 9 | 0.5 | 7 | 1 | 82.17 |
| 16 | 9 | 4 | 7 | 1 | 96.58 |
| 17 | 3 | 2.25 | 3 | 1 | 84.22 |
| 18 | 15 | 2.25 | 3 | 1 | 90.27 |
| 19 | 3 | 2.25 | 7 | 1 | 89.01 |
| 20 | 15 | 2.25 | 7 | 1 | 91.07 |
| 21 | 9 | 0.5 | 5 | 0.4 | 85.94 |
| 22 | 9 | 4 | 5 | 0.4 | 93.4 |
| 23 | 9 | 0.5 | 5 | 1.6 | 85.22 |
| 24 | 9 | 4 | 5 | 1.6 | 97.67 |
| 25 | 9 | 2.25 | 5 | 1 | 87.66 |
| 26 | 9 | 2.25 | 5 | 1 | 94.22 |
| 27 | 9 | 2.25 | 5 | 1 | 95.03 |
| 28 | 9 | 2.25 | 5 | 1 | 96.57 |
| 29 | 9 | 2.25 | 5 | 1 | 96.34 |
| Source | Std. Dev. | R2 | R2Adj | R2Pre | |
|---|---|---|---|---|---|
| Linear | 0.0005 | 0.5178 | 0.4731 | 0.3663 | Suggested |
| 2FI | 0.4283 | 0.5139 | 0.4793 | 0.2249 | |
| Quadratic | 0.0431 | 0.7643 | 0.6541 | 0.2930 | Suggested |
| Cubic | 0.5583 | 0.7844 | 0.6368 | −0.3889 | Aliased |
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 640.52 | 14 | 45.75 | 4.78 | 0.0030 | significant |
| A-P/G ratio | 146.16 | 1 | 146.16 | 15.27 | 0.0016 | |
| B-time | 195.29 | 1 | 195.29 | 20.41 | 0.0005 | |
| C-pH | 7.11 | 1 | 7.11 | 0.74 | 0.4031 | |
| D-formaldehyde | 76.15 | 1 | 76.15 | 7.96 | 0.0136 | |
| AB | 22.85 | 1 | 22.85 | 2.39 | 0.1446 | |
| AC | 3.98 | 1 | 3.98 | 0.42 | 0.5294 | |
| AD | 0.30 | 1 | 0.30 | 0.031 | 0.8627 | |
| BC | 53.73 | 1 | 53.73 | 5.62 | 0.0327 | |
| BD | 6.23 | 1 | 6.23 | 0.65 | 0.4334 | |
| CD | 3.44 | 1 | 3.44 | 0.36 | 0.5583 | |
| A2 | 103.67 | 1 | 103.67 | 10.83 | 0.0054 | |
| B2 | 41.08 | 1 | 41.08 | 4.29 | 0.0572 | |
| C2 | 9.82 | 1 | 9.82 | 1.03 | 0.3283 | |
| D2 | 4.12 | 1 | 4.12 | 0.43 | 0.5226 | |
| Residual | 133.96 | 14 | 9.57 | |||
| Lack of Fit | 80.58 | 10 | 8.06 | 0.60 | 0.7643 | not significant |
| Pure Error | 53.38 | 4 | 13.34 | |||
| Cor Total | 774.48 | 28 |
| No. | P/G Ratio | Time (h) | pH | Formaldehyde Dosage (mL/L) | Predicted Removal Rate % | Actual Removal Rate | |
|---|---|---|---|---|---|---|---|
| 1 | 11.170 | 2.692 | 4.614 | 0.419 | 97.716 | 96.611 | Selected |
| 2 | 15.000 | 2.250 | 5.000 | 1.600 | 95.451 | 95.172 | |
| 3 | 9.000 | 4.000 | 7.000 | 1.000 | 98.686 | 97.335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Wang, Y.; Zeng, W.; Xu, H.; Chen, B. Preparation of Heavy Metal Trapping Flocculant Polyacrylamide–Glutathione and Its Application for Cadmium Removal from Water. Polymers 2023, 15, 500. https://doi.org/10.3390/polym15030500
Ding W, Wang Y, Zeng W, Xu H, Chen B. Preparation of Heavy Metal Trapping Flocculant Polyacrylamide–Glutathione and Its Application for Cadmium Removal from Water. Polymers. 2023; 15(3):500. https://doi.org/10.3390/polym15030500
Chicago/Turabian StyleDing, Wenjie, Yunyan Wang, Weizhi Zeng, Hui Xu, and Bingxin Chen. 2023. "Preparation of Heavy Metal Trapping Flocculant Polyacrylamide–Glutathione and Its Application for Cadmium Removal from Water" Polymers 15, no. 3: 500. https://doi.org/10.3390/polym15030500