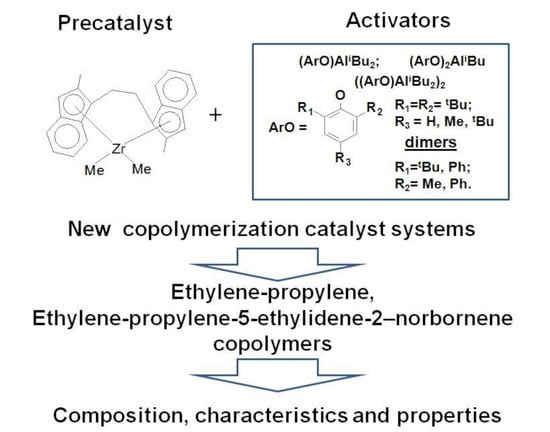
Synthesis and Properties of Ethylene/propylene and Ethylene/propylene/5-ethylidene-2-norbornene Copolymers Obtained on Rac-Et(2-MeInd)2ZrMe2/Isobutylaluminium Aryloxide Catalytic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Copolymerization of Ethylene and Propylene
2.3. Terpolymerization of Ethylene, Propylene and 5-Ethylidene-2-norbornene
2.4. Polymer Analysis
2.5. Dynamic Mechanical Analysis
2.6. Polymer Mechanical Tests
2.7. DFT Calculations
3. Results and Discussions
3.1. Copolymerization Results
3.2. Molecular Weight Characteristics, Crystallinity, and Thermal Properties of Copolymers
3.3. DMA Analysis of Copolymers
3.4. Mechanical Analysis of Copolymers
3.5. Thermooxidative Destruction of Copolymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ravishankar, P.S. Treatise on EPDM. Rubber Chem. Technol. 2012, 85, 327–349. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Faingol’d, E.E.; Badamshina, E.R.; Sanginov, E.A. Advances in synthesis of ethylene–propylene–diene elastomers by ion-coordination polymerization on single-site catalytic systems of new generation. Polym. Sci. Ser. C 2020, 62, 1–16. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Faingol’d, E.E.; Sanginov, E.A.; Badamshina, E.R. Homogeneous Group IVB Catalysts of New Generations for Synthesis of Ethylene-Propylene-Diene Rubbers: A Mini-Review. Catalysts 2022, 12, 704. [Google Scholar] [CrossRef]
- Van Duin, M.; van Doremaele, G.; van der Aar, N. Defining EPDM for the past and the next 50 years. KGK 2017, 11–12, 14–23. [Google Scholar]
- Global EPDM Market by Application (Automotive, Building & Construction, Plastic Modification, Tires & Tubes, Wires & Cables, Lubricant Additives) and Region (North America, Europe, Asia-Pacific, Middle East & Africa, South America)—Forecast to 2026. Available online: https://www.researchandmarkets.com/r/wnupt0 (accessed on 11 December 2022).
- Mahmood, Q.; Li, X.; Qin, L.; Wang, L.; Sun, W.-H. Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo)polymerization. Dalton Trans. 2022, 51, 14375–14407. [Google Scholar]
- Ge, Y.; Lu, Z.; Dai, S. Flexible Axial Shielding Strategy for the Synthesis of High-Molecular-Weight Polyethylene and Polar Functionalized Polyethylene with Pyridine-Imine Ni(II) and Pd(II) Complexes. Organometallics 2022, 41, 2042–2049. [Google Scholar] [CrossRef]
- Endo, K.; Hiwara, M.; Matsuura, S.; Mizobuchi, Y.; Yamamura, Y.; Noguchi, Y.; Ishii, Y.; Sakai, T.; Shishido, K.; Ichino, K.; et al. Ethylene Alpha-Olefin Non-Conjugated Polyene Copolymer, Use Thereof, and Manufacturing Method Thereof. U.S. Patent 0,347,894 A1, 1 December 2016. [Google Scholar]
- Ichino, K.; Kikuchi, Y.; Tohi, Y.; Matsugi, T.; Yanagimoto, Y.; Arino, M.; Shishido, K.; Hosoya, M. Ethylene/α-Olefin/Non-Conjugated Polyene Copolymer, and Production Process and Use Thereof. U.S. Patent 10,435,494 B2, 8 November 2018. [Google Scholar]
- Ichino, K.; Kikuchi, Y.; Shishido, K.; Tanaka, J.; Arino, M. Ethylene/Alpha-Olefin/Non-Conjugated Polyene Copolymer, Method for Producing the Same, and Use Thereof. U.S. Patent 0,009,730 A1, 14 January 2021. [Google Scholar]
- Starck, P.; Lehtinen, C.; Lofgren, B. Polymerization and characterization of ethylene/propylene and ethylene/1-octene copolymers produced with bridged Zr- and Hf-based metallocenes. Angew. Makromol. Chem. 1997, 249, 115–135. [Google Scholar] [CrossRef]
- Gillis, D.J.; Karpeles, R. Process for Producing Polyolefin Elastomer Employing a Metallocene Catalyst. U.S. Patent 6,225,426 B1, 1 May 2001. [Google Scholar]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Edit. 2014, 53, 9722–9744. [Google Scholar] [CrossRef] [Green Version]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene-α-olefin copolymerization reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef]
- Van Doremaele, G.; van Duin, M.; Valla, M.; Berthoud, A. On the development of titanium κ1-amidinate complexes, commercialized as Keltan ACE™ technology, enabling the production of an unprecedented large variety of EPDM polymer structures. J. Polym. Sci. Pol. Chem. 2017, 55, 2877–2891. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.; Shan, C.L.P.; Li, G.; Han, T.; Doelder, J.D. Sponge EPDM by design. Plast. Rubber Compos. 2019, 48, 32–41. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Bravaya, N.M.; Panin, A.N.; Saratovskikh, S.L.; Babkina, O.N. Use of Aryl Oxides of Isobutyl Aluminium as Activators Dialkylmetallocene Catalysts for Homopolymerisation Ethylene, Propylene, Copolymerisation of Ethylene with Propylene and Triple Copolymerisation of Ethylene, Propylene and Diene, Homogeneous Metallocene Catalyst Systems for Synthesis of Homo-and Copolymers of Olefins and Dienes, Method of Producing Homo- and Copolymers of Olefins and Dienes, Method for Stabilisation of Homo- and Copolymers of Olefins and Dienes. R.U. Patent 2,588,496 C2, 27 June 2016. [Google Scholar]
- Faingol’d, E.E.; Bravaya, N.M.; Panin, A.N.; Babkina, O.N.; Saratovskikh, S.L.; Privalov, V.I. Isobutylaluminum aryloxides as metallocene activators in homo- and copolymerization of olefins. J. Appl. Polym. Sci. 2016, 133, 43276. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Zharkov, I.V.; Bravaya, N.M.; Panin, A.N.; Saratovskikh, S.L.; Babkina, O.N.; Shilov, G.V. Sterically crowded dimeric diisobutylaluminum aryloxides: Synthesis, characteristics, and application as activators in homo- and copolymerization of olefins. J. Organomet. Chem. 2018, 871, 86–95. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Saratovskikh, S.L.; Panin, A.N.; Babkina, O.N.; Zharkov, I.V.; Garifullin, N.O.; Shilov, G.V.; Bravaya, N.M. Ethylene/propylene and ethylene/propylene/5-ethylidene-2-norbornene copolymerizations on metallocene/(2,6-tBu2PhO-)AliBu2 catalyst systems. Polymer 2021, 220, 123559. [Google Scholar] [CrossRef]
- Samuel, E.; Rausch, M.D. .pi.-Cyclopentadienyl and.pi.-indenyl compounds of titanium, zirconium, and hafnium containing.sigma.-bonded organic substituents. J. Am. Chem. Soc. 1973, 95, 6263–6267. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Faingol’d, E.E.; Babkina, O.N.; Panin, A.N.; Saratovskikh, S.L.; Zharkov, I.V.; Fushman, E.A. Syntheses of isobutylalumoxanes by triisobutylaluminum hydrolysis and their use as activators of dimethylated zirconocene in propylene polymerization. Russ. Chem. Bull. 2013, 62, 560–567. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Panin, A.N.; Faingol’d, E.E.; Saratovskikh, S.L.; Babkina, O.N.; Zharkov, I.V.; Perepelitsina, E.O. Isobutylalumoxanes as high-performance activators of rac-Et(2-MeInd)2ZrMe2 in copolymerization of ethylene with propylene and ternary copolymerization of ethylene, propylene, and 5-ethylidene-2-norbornene. Polym. Bull. 2016, 73, 473–491. [Google Scholar] [CrossRef]
- ASTM D3900-05a; Standard Test Methods for Rubber-Determination of Ethylene Units in Ethylene-Propylene Copolymers (EPM) and in Ethylene-Propylene-Diene Terpolymers (EPDM) by Infrared Spectrometry. ASTM: West Conshohocken, PA, USA, 2010.
- Zyabina, V.A.; Korobova, L.M.; Lifshits, I.A.; Novikova, N.N.; Nel’son, K.V. Determination of ethylene norbornene in ethylene, propylene, and ethylidene norbornene copolymers by means of IR spectroscopy. Zhurnal Prikl. Spektrosk. 1972, 17, 1048–1051. [Google Scholar]
- Cran, M.J.; Bigger, S.W. Quantitative Analysis of Polyethylene Blends by Fourier Transform Infrared Spectroscopy. Appl. Spectrosc. 2003, 57, 928–932. [Google Scholar] [CrossRef]
- Ammawath, W.Y.; Che Man, B.; Abdul Rahman, R.B.; Baharin, B.S. A fourier transform infrared spectroscopic method for determining butylated hydroxytoluene in palm olein and palm oil. J. Amer. Oil Chem. Soc. 2006, 83, 187–191. [Google Scholar] [CrossRef]
- Faingol’d, E.E.; Zharkov, I.V.; Bravaya, N.M.; Chernyak, A.V. Hydrolysis of isobutylaluminum aryloxides studied by 1H NMR and quantum chemical methods. Russ. Chem. Bull. 2016, 65, 1958–1965. [Google Scholar] [CrossRef]
- Marathe, S.; Sivaram, S. Regioselective copolymerization of 5-vinyl-2-norbornene with ethylene using zirconocene-methylaluminoxane catalysts: A facile route to functional polyolefins. Macromolecules 1994, 27, 1083–1086. [Google Scholar] [CrossRef]
- Ali, A.; Tufail, M.K.; Jamil, M.I.; Yaseen, W.; Iqbal, N.; Hussain, M.; Ali, A.; Aziz, T.; Fan, Z.; Guo, L. Comparative Analysis of Ethylene/Diene Copolymerization and Ethylene/Propylene/Diene Terpolymerization Using Ansa-Zirconocene Catalyst with Alkylaluminum/Borate Activator: The Effect of Conjugated and Nonconjugated Dienes on Catalytic Behavior and Polymer Microstructure. Molecules 2021, 26, 2037. [Google Scholar] [PubMed]
- Guerra, G.; De Ballesteros, O.R.; Venditto, V.; Galimberti, M.; Sartori, F.; Pucciariello, R. Pseudohexagonal Crystallinity and Thermal and Tensile Properties of Ethene–Propene Copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1095–1103. [Google Scholar] [CrossRef]
- Bracco, S.; Comotti, A.; Simonutti, R.; Camurati, I.; Sozzani, P. Low-Temperature Crystallization of Ethylene-ran-propylene Copolymers: Conformational Rearrangement of Sequences during the Formation of the Aggregates. Macromolecules 2002, 35, 1677–1684. [Google Scholar] [CrossRef]
- Hu, W.; Srinivas, S.; Sirota, E. Crystalline Structure and Properties of EP and EB Copolymers by Solid-State NMR, DSC, and WAXS. Macromolecules 2002, 35, 5013–5024. [Google Scholar] [CrossRef]
- Müller, A.J.; Arnal, M.L. Thermal fractionation of polymers. Prog. Polym. Sci. 2005, 30, 559–603. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Q.; Lü, T.; Huang, H.; He, T. Improved thermal fractionation technique for chain structure analysis of ethylene/α-olefin copolymers. Polymer 2002, 43, 1031–1034. [Google Scholar] [CrossRef]
- Matsko, M.A.; Vanina, M.P.; Echevskaya, L.G.; Zakharov, V.A. Study of the compositional heterogeneity of ethylene–hexene-1 copolymers by thermal fractionation technique by means of differential scanning calorimetry. J. Therm. Anal. Calorim. 2013, 113, 923–932. [Google Scholar] [CrossRef]
- Matsko, M.A.; Semikolenova, N.V.; Zakharov, V.A.; Soshnikov, I.E.; Shundrina, I.K.; Sun, W.-H. Formation of branched polyethylenes by ethylene homopolymerization using LNiBr2 homo- and heterogeneous precatalysts: Interpretation of the polymer structures in comparison with commercial LLDPE. J. Appl. Polym. Sci. 2021, 138, 50436. [Google Scholar] [CrossRef]
- Pospíšil, J. Mechanistic action of phenolic antioxidants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Roginskiy, V.A. Phenolic Antioxidants: Reactive Capacity and Effectiveness; Nauka: Moscow, Russia, 1988. (In Russian) [Google Scholar]






| № | Activator | M a) | A b) | Mw | Mw/Mn | Copolymer Composition E/P/ENB mol. % | Tm c), °C | ∆H d), J/g | χ e), % X-ray | σr f), MPa | εrg), % | EL h) % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1-DTBP | E/P | 92.4 | 89,000 | 2.7 | 86/14/- | 101 | 71.5 | 39 | 5.0 | 600 | 130 |
| 2 | 1-BHT | E/P | 79.6 | 108,000 | 2.7 | 85/15/- | 96 | 59.9 | 33 | 5.2 | 850 | 190 |
| 3 k) | 1-TTBP | E/P | 74.4 | 143,000 | 2.3 | 91/9/- | 102 | 88.6 | 37 | 15.8 | 840 | 610 |
| 4 k) | 2-DTBP | E/P | 36.6 | 96,000 | 2.2 | 89/11/- | 99 | 77.0 | 36 | 9.0 | 700 | 500 |
| 5 k) | 2-BHT | E/P | 32.8 | 160,000 | 2.3 | 92/8/- | 101 | 95.2 | 42 | 25.0 | 1050 | 820 |
| 6 l) | 1-MTBP (dimer) | E/P | 85.8 | 98,000 | 3.5 | 86/14/- | 102 | 68.8 | 35 | 3.0 | 290 | - |
| 7 l) | 1-DPP (dimer) | E/P | 48.6 | 105,000 | 2.5 | 88/12/- | 103 | 70.9 | 42 | 13.4 | 900 | 740 |
| 8 k) | 1-DTBP | E/P/ENB | 39.2 | 111,000 | 2.6 | 85/13/2 | 85, 101 | 58.8 | 21 | 21.6 | 560 | 210 |
| 9 k) | 1-BHT | E/P/ENB | 35.2 | 115,000 | 3.0 | 88/8/4 | 81, 106 | 57.7 | 21 | 18.0 | 520 | 230 |
| 10 k) | 1-TTBP | E/P/ENB | 32.2 | n.s.i) | - | 90/8/2 | 85, 101, 120 | 56.7 | 24 | 11.0 | 200 | 130 |
| 11 k) | 2-DTBP | E/P/ENB | 9.2 | 176,000 | 2.3 | 91/7/2 | 87 | 56.7 | 27 | 14.0 | 400 | 170 |
| 12 k) | 2-BHT | E/P/ENB | 8.6 | 181,000 | 2.2 | 93/5/2 | 83, 99, 122 | 57.0 | 27 | 7.0 k) | 100 j) | - |
| 13 l) | 1-MTBP (dimer) | E/P/ENB | 19.6 | 165,000 | 2.2 | 91/7/2 | 90, 105 | 64.3 | - | 13.7 | 290 | 210 |
| 14 l) | 1-DPP (dimer) | E/P/ENB | 7.6 | n.s.i) | - | 91/6/3 | 44, 86, 98 | 55.1 | 32 | 19.6 | 570 | 320 |
| № a) | E/P Content Mol. % b) | Activator | Peak Ratio c) | ∑ of Three Peaks, d) % |
|---|---|---|---|---|
| 1 Peak/2 Peak/3 Peak (Tm) | ||||
| 1 | 14/0 | 1-DTBP | 0.75(79)/0.90 (89)/1.00 (99) | 63 |
| 2 | 15/0 | 1-BHT | 0.79 (79)/1.00 (89)/0.76 (99) | 48 |
| 3 | 9/0 | 1-TTBP | 0.50 (82)/0.66 (91)/1.00 (101) | 74 |
| 4 | 11/0 | 2-DTBP | 0.64 (80)/0.86 (90)/1.00 (100) | 64 |
| 5 | 8/0 | 2-BHT | 0.40 (81)/0.68 (92)/1.00 (101) | 75 |
| 6 | 14/0 | 1-MTBP | 0.35 (70)/0.36 (80)/1.00 (90) | 66 |
| 9 | 8/4 | 1-BHT | 0.78 (69)/1.00 (78)/0.65 (87) | 57 |
| 10 | 8/2 | 1-TTBP | 0.58 (70)/1.00 (81)/0.96 (89) | 66 |
| 11 | 7/2 | 2-DTBP | 0.57 (68)/0.99 (78)/1.00 (87) | 74 e) |
| № * | Activator | Copolymer | E′ (−70 °C), MPa | E′ (0 °C), MPa | PeakE″, MPa | Tg (E′), °C | Tg (E″), °C |
|---|---|---|---|---|---|---|---|
| 1 | 1-DTBP | E/P | 1469 | 190 | 91 | −51 | −57 |
| 6 | 1-MTBP | E/P | 1495 | 190 | 95 | −49 | −53 |
| 4 | 2-DTBP | E/P | 1095 | 190 | 59 | −38 | −40 |
| 8 | 1-DTBP | E/P/ENB | 1537 | 311 | 106 | −8 | −8 |
| 11 | 2-DTBP | E/P/ENB | 1575 | 433 | 118 | −11 | −11 |
| Activator | T (∆m = 5%), °C | T (∆m = 10%), °C | T (∆m = 50%), °C |
|---|---|---|---|
| IBAO | 290 | 335 | 411 |
| 1-BHT | 313 | 340 | 436 |
| 1-MTBP | 316 | 353 | 449 |
| 1-DTBP | 313 | 355 | 453 |
| 1-TTBP | 340 | 400 | 456 |
| 1-DPP | 410 | 419 | 467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faingol’d, E.E.; Saratovskikh, S.L.; Panin, A.N.; Babkina, O.N.; Zharkov, I.V.; Kapasharov, A.T.; Bubnova, M.L.; Shilov, G.V.; Bravaya, N.M. Synthesis and Properties of Ethylene/propylene and Ethylene/propylene/5-ethylidene-2-norbornene Copolymers Obtained on Rac-Et(2-MeInd)2ZrMe2/Isobutylaluminium Aryloxide Catalytic Systems. Polymers 2023, 15, 487. https://doi.org/10.3390/polym15030487
Faingol’d EE, Saratovskikh SL, Panin AN, Babkina ON, Zharkov IV, Kapasharov AT, Bubnova ML, Shilov GV, Bravaya NM. Synthesis and Properties of Ethylene/propylene and Ethylene/propylene/5-ethylidene-2-norbornene Copolymers Obtained on Rac-Et(2-MeInd)2ZrMe2/Isobutylaluminium Aryloxide Catalytic Systems. Polymers. 2023; 15(3):487. https://doi.org/10.3390/polym15030487
Chicago/Turabian StyleFaingol’d, Evgeny E., Stanislav L. Saratovskikh, Andrei N. Panin, Olga N. Babkina, Igor V. Zharkov, Artur T. Kapasharov, Maria L. Bubnova, Gennady V. Shilov, and Natalia M. Bravaya. 2023. "Synthesis and Properties of Ethylene/propylene and Ethylene/propylene/5-ethylidene-2-norbornene Copolymers Obtained on Rac-Et(2-MeInd)2ZrMe2/Isobutylaluminium Aryloxide Catalytic Systems" Polymers 15, no. 3: 487. https://doi.org/10.3390/polym15030487