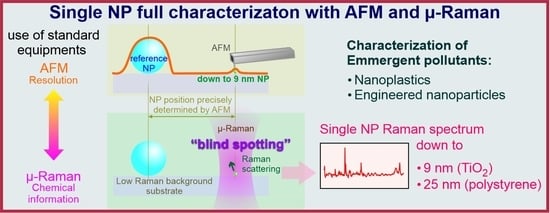
Sub-10 nm Nanoparticle Detection Using Multi-Technique-Based Micro-Raman Spectroscopy
Abstract
:1. Introduction
AFM/MRS Precision Colocalization Technique
2. Materials and Methods
2.1. Nanoparticles (NPs)
2.2. Substrates
2.3. Solution Preparation and Deposition
2.4. Micro-Raman Spectroscopy (MRS)
2.5. Atomic Force Microscopy (AFM)
3. Results and Discussion
3.1. Visualization and Raman Acquisition of Large NPLs
3.2. Direct, Blind Targeting of NPLs
3.3. Direct, Blind Targeting of TiO2 NPs
3.4. Signal to Noise Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Low Raman Background Noise Substrates for Single-Particle Detection

Appendix B. Characterization of Stage Drift

Appendix C. Colocalized Raman Map for the PS NPs of Intermediate Sizes

References
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Sinclair, C.J.; Bradley, E.L.; Boxall, A.B.A. Effects of Environmental Conditions on Latex Degradation in Aquatic Systems. Sci. Total Environ. 2013, 447, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Hansson, L.-A.; Cedervall, T. Nano-Plastics in the Aquatic Environment. Environ. Sci. Process. Impacts 2015, 17, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics Formed during the Mechanical Breakdown of Daily-Use Polystyrene Products. Nanoscale Adv. 2019, 1, 1055–1061. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of Nanoplastics during the Degradation of Polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and Challenges in the Detection of Microplastics and Nanoplastics: A Mini-Review. Polym. Int. 2022, 71, 543–551. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and Nanoplastics Analysis: Options, Imaging, Advancements and Challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical Methods for Microplastics in the Environment: A Review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Ladd, J.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Label-Free Detection of Cancer Biomarker Candidates Using Surface Plasmon Resonance Imaging. Anal. Bioanal. Chem. 2009, 393, 1157–1163. [Google Scholar] [CrossRef]
- Caldwell, J.; Taladriz-Blanco, P.; Lehner, R.; Lubskyy, A.; Ortuso, R.D.; Rothen-Rutishauser, B.; Petri-Fink, A. The Micro-, Submicron-, and Nanoplastic Hunt: A Review of Detection Methods for Plastic Particles. Chemosphere 2022, 293, 133514. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production As a Basis for Exposure Assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial Production Quantities and Uses of Ten Engineered Nanomaterials in Europe and the World. J. Nanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Biswas, P.; Wu, C.-Y. Nanoparticles and the Environment. J. Air Waste Manag. Assoc. 2005, 55, 708–746. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825. [Google Scholar] [CrossRef] [PubMed]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Wang, S.Y.; Lin, S.T.; Kung, A.H. Sub-Nanosecond Timing Jitter in a Passively Q-Switched Microlaser by Active Q-Switched Laser Bleaching. In Proceedings of the 2013 Conference on Lasers and Electro-Optics Pacific Rim (CLEOPR), Kyoto, Japan, 30 June–4 July 2013; pp. 2–3. [Google Scholar] [CrossRef]
- Kung, H.-C.; Wu, C.-H.; Cheruiyot, N.K.; Mutuku, J.K.; Huang, B.-W.; Chang-Chien, G.-P. The Current Status of Atmospheric Micro/Nanoplastics Research: Characterization, Analytical Methods, Fate, and Human Health Risk. Aerosol Air Qual. Res. 2023, 23, 220362. [Google Scholar] [CrossRef]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the Analysis of Submicrometer- and Nanoplastic Particles in the Environment. TrAC Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Vitali, C.; Peters, R.J.B.; Janssen, H.G.; Nielen, M.W.F.; Ruggeri, F.S. Microplastics and Nanoplastics in Food, Water, and Beverages, Part II. Methods. TrAC Trends Anal. Chem. 2022, 157, 116819. [Google Scholar] [CrossRef]
- Mandemaker, L.D.B.; Meirer, F. Spectro-Microscopic Techniques for Studying Nanoplastics in the Environment and in Organisms. Angew. Chem. Int. Ed. 2023, 62, e202210494. [Google Scholar] [CrossRef]
- Hassoun, A.; Pasti, L.; Chenet, T.; Rusanova, P.; Smaoui, S.; Aït-Kaddour, A.; Bono, G. Detection Methods of Micro and Nanoplastics. In Advances in Food and Nutrition Research; Elsevier, Inc.: Amsterdam, The Netherlands, 2023; Volume 103, pp. 175–227. ISBN 9780323988353. [Google Scholar]
- Anderson, N.; Anger, P.; Hartschuh, A.; Novotny, L. Subsurface Raman Imaging with Nanoscale Resolution. Nano Lett. 2006, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Micic, M.; Klymyshyn, N.; Suh, Y.D.; Lu, H.P. Finite Element Method Simulation of the Field Distribution for AFM Tip-Enhanced Surface-Enhanced Raman Scanning Microscopy. J. Phys. Chem. B 2003, 107, 1574–1584. [Google Scholar] [CrossRef]
- Meyns, M.; Primpke, S.; Gerdts, G. Library Based Identification and Characterisation of Polymers with Nano-FTIR and IR-SSNOM Imaging. Anal. Methods 2019, 11, 5195–5202. [Google Scholar] [CrossRef]
- Lu, F.; Belkin, M.A. Infrared Absorption Nano-Spectroscopy Using Sample Photoexpansion Induced by Tunable Quantum Cascade Lasers. Opt. Express 2011, 19, 19942. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Morrison, W.; Wickramasinghe, H.K.; Jahng, J.; Potma, E.; Wan, L.; Ruiz, R.; Albrecht, T.R.; Schmidt, K.; Frommer, J.; et al. Nanoscale Chemical Imaging by Photoinduced Force Microscopy. Sci. Adv. 2016, 2, 4–7. [Google Scholar] [CrossRef]
- Yeo, B.S.; Amstad, E.; Schmid, T.; Stadler, J.; Zenobi, R. Nanoscale Probing of a Polymer-Blend Thin Film with Tip-Enhanced Raman Spectroscopy. Small 2009, 5, 952–960. [Google Scholar] [CrossRef]
- ten Have, I.C.; Duijndam, A.J.A.; Oord, R.; Berlo-van den Broek, H.J.M.; Vollmer, I.; Weckhuysen, B.M.; Meirer, F. Photoinduced Force Microscopy as an Efficient Method Towards the Detection of Nanoplastics. Chem. Methods 2021, 1, 205–209. [Google Scholar] [CrossRef]
- Meirer, F.; ten Have, I.C.; Oord, R.; Zettler, E.R.; van Sebille, E.; Amaral-Zettler, L.A.; Weckhuysen, B.M. Nanoscale Infrared Spectroscopy Reveals Nanoplastics at 5000 m Depth in the South Atlantic Ocean. Res. Squre 2021, 1–14. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Ajito, K.; Torimitsu, K. Single Nanoparticle Trapping Using a Raman Tweezers Microscope. Appl. Spectrosc. 2002, 56, 541–544. [Google Scholar] [CrossRef]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman Tweezers for Small Microplastics and Nanoplastics Identification in Seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Sobhani, Z.; Zhang, X.; Gibson, C.T.; Tang, Y.; Naidu, R. Identification and Visualisation of Microplastics/Nanoplastics by Raman Imaging (ii): Smaller than the Diffraction Limit of Laser? Water Res. 2020, 183, 116046. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, K.; Wang, W.; Wei, L.; Lai, Y. Quantitative and Sensitive Analysis of Polystyrene Nanoplastics down to 50 Nm by Surface-Enhanced Raman Spectroscopy in Water. J. Hazard. Mater. 2022, 429, 128388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Liu, R.; Hao, L.T.; Liu, J.F. Identification of Polystyrene Nanoplastics Using Surface Enhanced Raman Spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Yu, E.S.; Jeong, E.T.; Lee, S.; Kim, I.S.; Chung, S.; Han, S.; Choi, I.; Ryu, Y.S. Real-Time Underwater Nanoplastic Detection beyond the Diffusion Limit and Low Raman Scattering Cross-Section via Electro-Photonic Tweezers. ACS Nano 2023, 17, 2114–2123. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Su, J.; Li, S.; Lv, X.; Chen, J.; Lai, Y.; Zhan, J. Identification of Trace Polystyrene Nanoplastics Down to 50 Nm by the Hyphenated Method of Filtration and Surface-Enhanced Raman Spectroscopy Based on Silver Nanowire Membranes. Environ. Sci. Technol. 2022, 56, 10818–10828. [Google Scholar] [CrossRef]
- Fang, C.; Sobhani, Z.; Zhang, X.; McCourt, L.; Routley, B.; Gibson, C.T.; Naidu, R. Identification and Visualisation of Microplastics/Nanoplastics by Raman Imaging (iii): Algorithm to Cross-Check Multi-Images. Water Res. 2021, 194, 116913. [Google Scholar] [CrossRef]
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and Visualisation of Microplastics by Raman Mapping. Anal. Chim. Acta 2019, 1077, 191–199. [Google Scholar] [CrossRef]
- Bolduc, E.; Faccio, D.; Leach, J. Acquisition of Multiple Photon Pairs with an EMCCD Camera. J. Opt. 2017, 19, 054006. [Google Scholar] [CrossRef]
- Dieing, T.; Hollricher, O. High-Resolution, High-Speed Confocal Raman Imaging. Vib. Spectrosc. 2008, 48, 22–27. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]











| Detection Technique | TNP | RNP | TNP Concentration ng·mL−1 | RNP Concentration ng·mL−1 |
|---|---|---|---|---|
| Visual | PS 500 nm | None | 10 × 106 | |
| Visual | PS 200 nm | None | 5 × 106 | |
| Visual | PS 100 nm | None | 2 × 106 | |
| Visual | PS 50 nm | None | 1 × 106 | |
| Mapping | PS 50 nm | PS 200 nm | 50 | 300 |
| Blind | PS 25 nm | PS 200 nm | 9 | 300 |
| Blind | TiO2 20 nm | TiO2 400 nm | 15 | 10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bereczki, A.; Dipold, J.; Freitas, A.Z.; Wetter, N.U. Sub-10 nm Nanoparticle Detection Using Multi-Technique-Based Micro-Raman Spectroscopy. Polymers 2023, 15, 4644. https://doi.org/10.3390/polym15244644
Bereczki A, Dipold J, Freitas AZ, Wetter NU. Sub-10 nm Nanoparticle Detection Using Multi-Technique-Based Micro-Raman Spectroscopy. Polymers. 2023; 15(24):4644. https://doi.org/10.3390/polym15244644
Chicago/Turabian StyleBereczki, Allan, Jessica Dipold, Anderson Z. Freitas, and Niklaus U. Wetter. 2023. "Sub-10 nm Nanoparticle Detection Using Multi-Technique-Based Micro-Raman Spectroscopy" Polymers 15, no. 24: 4644. https://doi.org/10.3390/polym15244644