Effect of the Addition of Fique Bagasse Cellulose Nanoparticles on the Mechanical and Structural Properties of Plastic Flexible Films from Cassava Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
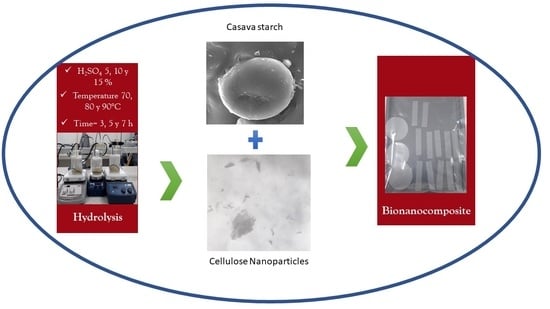
2.2. Production of Cellulose Nanoparticles (CNCs)
2.3. High-Resolution Optical Microscopy
2.4. Electron Transmission Microscopy (TEM)
2.5. Color Properties
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Film Preparation
2.8. Thermal Properties
2.8.1. Differential Scanning Calorimetry (DCS)
2.8.2. Thermogravimetric Analysis (TGA)
2.9. Evaluation of Mechanical Properties
2.10. Bionanocomposite Matrix Barrier Properties
2.11. Scanning Electron Microscopy (SEM)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Variation of Particle Size in the Acid Hydrolysis Process
3.2. Size Particle Measurement by TEM
3.3. Structural Changes by FTIR of the FB in the Process of Obtaining Nanoparticles
3.4. Effect of Temperature on Color Properties of Nanoparticles with Respect to Processing Time and Acid Concentration
3.5. Mechanical Properties of the Bionanocomposite Matrix
3.6. Gas Oxygen Permeability of TPS Films with CNC Addition
3.7. Effect of the CNCs on the Structural Change in the Starch Film
3.8. Analysis of Thermal Properties of the CNCs and the Bionanocomposite Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilares, R.T.; Resende, J.; Orsi, C.; Ahmed, M.; Lacerda, T.; da Silva, S.; Santos, J. Exopolysaccharide (pullulan) production from sugarcane bagasse hydrolysate aiming to favor the development of biorefineries. Int. J. Biol. Macromol. 2019, 127, 169–177. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide. Polymers 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Saari, U.; Fedoruk, M.; Iital, A.; Moora, H.; Klöga, M.; Voronova, V. An overview of the problems posed by plastic products and the role of extended producer responsibility in Europe. J. Clean. Prod. 2019, 214, 550–558. [Google Scholar] [CrossRef]
- Sathish, N.; Jeyasanta, K.I.; Patterson, J. Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Mar. Pollut. Bull. 2019, 142, 112–118. [Google Scholar] [CrossRef]
- Foschi, E.; Bonoli, A. The Commitment of Packaging Industry in the Framework of the European Strategy for Plastics in a Circular Economy. Adm. Sci. 2019, 9, 18. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef]
- Djekic, I.; Miloradovic, Z.; Djekic, S.; Tomasevic, I. Household food waste in Serbia—Attitudes, quantities and global warming potential. J. Clean. Prod. 2019, 229, 44–52. [Google Scholar] [CrossRef]
- Maga, D.; Hiebel, M.; Aryan, V. A Comparative Life Cycle Assessment of Meat Trays Made of Various Packaging Materials. Sustainability 2019, 11, 5324. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Kulikowska, D.; Bernat, K. Effect of Bio-Based Products on Waste Management. Sustainability 2020, 12, 2088. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.; Laycock, B. Environmental impact of biodegradable food packaging when considering food waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- Ávila-Orta, C.A.; Corral, F.S.; Fonseca-Florido, H.A.; Aguilar, F.I.E.; Rosales, S.G.S.; Padilla, J.M.M.; Morones, P.G.; Tavizón, S.F.; Hernández-Hernández, E. Starch-graphene oxide bionanocomposites prepared through melt mixing. J. Appl. Polym. Sci. 2018, 135, 46037. [Google Scholar] [CrossRef]
- Noshirvani, N.; Hong, W.; Ghanbarzadeh, B.; Fasihi, H.; Montazami, R. Study of cellulose nanocrystal doped starch-polyvinyl alcohol bionanocomposite films. Int. J. Biol. Macromol. 2018, 107, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, P.; Zhou, M.; Guo, R.; Cheng, F.; Lin, Y.; Yang, R. Microfibrillated cellulose modified with urea and its reinforcement for starch-based bionanocomposites. Cellulose 2019, 26, 5981–5993. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A.; Mahardika, M.; Hafizulhaq, F. Mechanical Properties of a Water Hyacinth Nanofiber Cellulose Reinforced Thermoplastic Starch Bionanocomposite: Effect of Ultrasonic Vibration during Processing. Fibers 2018, 6, 40. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Abdolshahi, A.; Dahmardeh, S.; Mohammadi, M.M. Poly(lactic acid)-based bionanocomposites: Effects of ZnO nanoparticles and essential oils on physicochemical properties. Polym. Bull. 2022, 79, 97–119. [Google Scholar] [CrossRef]
- Doh, H.; Dunno, K.D.; Whiteside, W.S. Cellulose nanocrystal effects on the biodegradability with alginate and crude seaweed extract nanocomposite films. Food Biosci. 2020, 38, 100795. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of functionalized silica nanoparticles on the mass transfer process in active PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Magnani, C.; Idström, A.; Nordstierna, L.; Müller, A.J.; Dubois, P.; Raquez, J.-M.; Re, G.L. Interphase Design of Cellulose Nanocrystals/Poly(hydroxybutyrate-ran-valerate) Bionanocomposites for Mechanical and Thermal Properties Tuning. Biomacromolecules 2020, 21, 1892–1901. [Google Scholar] [CrossRef]
- Ben Hamou, K.; Kadimi, A.; Meri, R.M.; Gaidukov, S.; Kaddami, H.; Raihane, M.; Lahcini, M.; Erchiqui, F. Synergistic Effect of Halloyosite Nanotube and Nanocellulose on Thermal and Mechanical Properties of Poly (Ethylmethacrylate-co-Acrylonitrile) Bionanocomposites. J. Renew. Mater. 2020, 8, 301–317. [Google Scholar] [CrossRef]
- Salam, H.; Dong, Y.; Davies, I.J.; Pramanik, A. Optimization of material formulation and processing parameters in relation to mechanical properties of bioepoxy/clay nanocomposites using Taguchi design of experiments. J. Appl. Polym. Sci. 2018, 135, 45769. [Google Scholar] [CrossRef]
- Bagheri, V.; Ghanbarzadeh, B.; Ayaseh, A.; Ostadrahimi, A.; Ehsani, A.; Alizadeh-Sani, M.; Adun, P.A. The optimization of physico-mechanical properties of bionanocomposite films based on gluten/ carboxymethyl cellulose/cellulose nanofiber using response surface methodology. Polym. Test. 2019, 78, 749–758. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Varghese, T.O.; Nayak, S.K. Isolation and characterisation of nanofibrillated cellulose from waste cotton: Effects on thermo-mechanical properties of polylactic acid/MA-g-SEBS blends. Iran. Polym. J. 2019, 28, 673–683. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Qian, S.; Lopez, C.A.F. High-toughness PLA/Bamboo cellulose nanowhiskers bionanocomposite strengthened with silylated ultrafine bamboo-char. Compos. Part B Eng. 2019, 165, 174–182. [Google Scholar] [CrossRef]
- da Silva, C.G.; Kano, F.S.; Rosa, D.S. Lignocellulosic Nanofiber from Eucalyptus Waste by a Green Process and Their Influence in Bionanocomposites. Waste Biomass Valorization 2020, 11, 3761–3774. [Google Scholar] [CrossRef]
- Monteiro, S.N.; de Assis, F.S.; Ferreira, C.L.; Simonassi, N.T.; Weber, R.P.; Oliveira, M.S.; Colorado, H.A.; Pereira, A.C. Fique Fabric: A Promising Reinforcement for Polymer Composites. Polymers 2018, 10, 246. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; El-Sayed, H.S.; El-Sayed, S.M.; Elaaser, M.; El-Salam, M.H.A. Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Adv. 2020, 10, 37857–37870. [Google Scholar] [CrossRef]
- MADR, “CADENA DEL FIQUE Y SU AGROINDUSTRIA” – Indicadores. MINAGRICULTURA 2018. Bogotá, Colombia. Available online: https://sioc.minagricultura.gov.co/Fique/Documentos/2018-11-30%20Cifras%20Sectoriales.pdf (accessed on 10 February 2023).
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef]
- Lago, R.C.D.; de Oliveira, A.L.M.; Dias, M.C.; de Carvalho, E.E.N.; Tonoli, G.H.D.; Boas, E.V.D.B.V. Obtaining cellulosic nanofibrils from oat straw for biocomposite reinforcement: Mechanical and barrier properties. Ind. Crops Prod. 2020, 148, 112264. [Google Scholar] [CrossRef]
- Kazharska, M.; Ding, Y.; Arif, M.; Jiang, F.; Cong, Y.; Wang, H.; Zhao, C.; Liu, X.; Chi, Z.; Liu, C. Cellulose nanocrystals derived from Enteromorpha prolifera and their use in developing bionanocomposite films with water-soluble polysaccharides extracted from E. prolifera. Int. J. Biol. Macromol. 2019, 134, 390–396. [Google Scholar] [CrossRef]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Palechor-Tróchez, J.J.; Santos, L.E.O.; Villada-Castillo, H.S. Relationship between Color CIELab and Total Organic Carbon in Compost. Adv. Mater. Sci. Eng. 2018, 2018, 5474239. [Google Scholar] [CrossRef]
- Imbachi-Hoyos, R.C.; Salazar-Sánchez, M.D.R.; Solanilla-Duque, J.F. Study of morphological, structural, and thermal changes of biodegradable material in simulated natural environmental conditions. Environ. Qual. Manag. 2022, 32, 365–378. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R.; Zainudin, E.S. Sugar palm nanocrystalline cellulose reinforced sugar palm starch composite: Degradation and water-barrier properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012006. [Google Scholar] [CrossRef]
- Acosta, D.F.R.; Duque, J.F.S.; LaVerde, L.M.A.; Castillo, H.S.V.; Tolaba, M.P. Structural and thermal properties of the amaranth starch granule obtained by high-impact wet milling. Int. J. Food Eng. 2020, 16, 20200024. [Google Scholar] [CrossRef]
- Arboleda, G.A.; Montilla, C.E.; Villada, H.S.; Varona, G.A. Obtaining a Flexible Film Elaborated from Cassava Thermoplastic Starch and Polylactic Acid. Int. J. Polym. Sci. 2015, 2015, 627268. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y.; Han, N.; Liu, S. Water and gas barrier properties of polyvinyl alcohol (PVA)/starch (ST)/ glycerol (GL)/halloysite nanotube (HNT) bionanocomposite films: Experimental characterisation and modelling approach. Compos. Part B: Eng. 2019, 174, 107033. [Google Scholar] [CrossRef]
- Alrawi, L.I.; Noriman, N.; Alomar, M.K.; Alakrach, A.M.; Dahham, O.S.; Hamzah, R.; Betar, B.O.; Jassam, T.M. Tensile and Morphology Properties of PLA/MMT-TiO2 Bionanocomposites. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2020; pp. 131–135. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II nanocrystals produced by sulfuric acid hydrolysis of Tetra pak cellulose I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Soy protein isolate nanocomposites reinforced with nanocellulose isolated from licorice residue: Water sensitivity and mechanical strength. Ind. Crops Prod. 2018, 117, 252–259. [Google Scholar] [CrossRef]
- Khoo, R.; Ismail, H.; Chow, W. Thermal and Morphological Properties of Poly (Lactic Acid)/Nanocellulose Nanocomposites. Procedia Chem. 2016, 19, 788–794. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, L.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with a triazine derivative and their reinforcement of poly(lactic acid)-based bionanocomposites. Cellulose 2018, 25, 2965–2976. [Google Scholar] [CrossRef]
- Robles, E.; Fernández-Rodríguez, J.; Barbosa, A.M.; Gordobil, O.; Carreño, N.L.; Labidi, J. Production of cellulose nanoparticles from blue agave waste treated with environmentally friendly processes. Carbohydr. Polym. 2018, 183, 294–302. [Google Scholar] [CrossRef]
- Rohner, S.; Humphry, J.; Chaléat, C.M.; Vandi, L.-J.; Martin, D.J.; Amiralian, N.; Heitzmann, M.T. Mechanical properties of polyamide 11 reinforced with cellulose nanofibres from Triodia pungens. Cellulose 2018, 25, 2367–2380. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using Acid hydrolysis. Int. J. Biol. Macromol. 2018, 107, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Gañán, P.; Mondragon, I. Surface Modification of Fique Fibers. Effects on Their Physico-Mechanical Properties. Polym. Compos. 2002, 23, 383–394. [Google Scholar] [CrossRef]
- Gañań, P.; Mondragon, I. Effect of Fiber Treatments on Mechanical Behavior of Short Fique Fiber-reinforced Polyacetal Composites. J. Compos. Mater. 2005, 39, 633–646. [Google Scholar] [CrossRef]
- Adel, A.M.; El–Wahab, Z.H.A.; Ibrahim, A.A.; Al–Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef] [PubMed]
- Aqlil, M.; Nzenguet, A.M.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene Oxide Filled Lignin/Starch Polymer Bionanocomposite: Structural, Physical, and Mechanical Studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.G.; Vázquez, A. Flexural properties loss of unidirectional epoxy/fique composites immersed in water and alkaline medium for construction application. Compos. Part B Eng. 2012, 43, 3120–3130. [Google Scholar] [CrossRef]
- Hoyos, C.G.; Alvarez, V.A.; Rojo, P.G.; Vázquez, A. Fique fibers: Enhancement of the tensile strength of alkali treated fibers during tensile load application. Fibers Polym. 2012, 13, 632–640. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Rusu, D.; Darie-Niţă, R.N.; Tîmpu, D.; Ciolacu, F. Influence of Gel Stage from Cellulose Dissolution in NaOH-Water System on the Performances of Cellulose Allomorphs-Based Hydrogels. Gels 2022, 8, 410. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Ovalle-Serrano, S.; Gómez, F.; Blanco-Tirado, C.; Combariza, M. Isolation and characterization of cellulose nanofibrils from Colombian Fique decortication by-products. Carbohydr. Polym. 2018, 189, 169–177. [Google Scholar] [CrossRef]
- Reddy, J.P.; Rhim, J.-W. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr. Polym. 2014, 110, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 2011, 18, 443–450. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sain, M.; Kortschot, M. Reinforcing potential of wood pulp-derived microfibres in a PVA matrix. Holzforschung 2006, 60, 53–58. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H. Bio-nanocomposite Materials for Food Packaging Applications: Types of Biopolymer and Nano-sized Filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Ambrosio-Martín, J.; Lagaron, J.M. Improving the barrier properties of thermoplastic corn starch-based films containing bacterial cellulose nanowhiskers by means of PHA electrospun coatings of interest in food packaging. Food Hydrocoll. 2016, 61, 261–268. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Rifici, S.; Botta, L.; Morreale, M.; La Mantia, F.P. Rheological and mechanical properties of biodegradable nanocomposites. AIP Conf. Proc. 2018, 1981, 020062. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Asad, M.; Saba, N.; Asiri, A.M.; Jawaid, M.; Indarti, E.; Wanrosli, W. Preparation and characterization of nanocomposite films from oil palm pulp nanocellulose/poly (Vinyl alcohol) by casting method. Carbohydr. Polym. 2018, 191, 103–111. [Google Scholar] [CrossRef]
- Vaezi, K.; Asadpour, G.; Sharifi, S.H. Bio nanocomposites based on cationic starch reinforced with montmorillonite and cellulose nanocrystals: Fundamental properties and biodegradability study. Int. J. Biol. Macromol. 2020, 146, 374–386. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Kim, E.-S.; Shim, W.-G.; Yoon, S.-D. Physical properties of mungbean starch/PVA bionanocomposites added nano-ZnS particles and its photocatalytic activity. J. Ind. Eng. Chem. 2018, 68, 57–68. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Carvalho, A. TPS Nanocomposite reinforced with MFC by melting process. Mater. Res. 2014, 17, 807–810. [Google Scholar] [CrossRef]
- Dehnad, D.; Emam-Djomeh, Z.; Mirzaei, H.; Jafari, S.-M.; Dadashi, S. Optimization of physical and mechanical properties for chitosan–nanocellulose biocomposites. Carbohydr. Polym. 2014, 105, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Ghanbarzadeh, B.; Milani, J.M.; Hamishehkar, H. Development of Gelatin Bionanocomposite Films Containing Chitin and ZnO Nanoparticles. Food Bioprocess Technol. 2017, 10, 1441–1453. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Preparation and characterization of sodium carboxymethyl cellulose/cotton linter cellulose nanofibril composite films. Carbohydr. Polym. 2015, 127, 101–109. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Sengupta, S. Study of the Properties of Microcrystalline Cellulose Particles from Different Renewable Resources by XRD, FTIR, Nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. [Google Scholar] [CrossRef]
- Iggui, K.; Kaci, M.; Le Moigne, N.; Bergeret, A. The Effects of Accelerated Photooxidation on Molecular Weight and Thermal and Mechanical Properties of PHBV/Cloisite 30B Bionanocomposites. J. Renew. Mater. 2017, 6, 288–298. [Google Scholar] [CrossRef]
- KPiyada; Waranyou, S.; Thawien, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]













| Sample | Tensile Modulus | Tensile Stress | Elongation |
|---|---|---|---|
| (MPa) | (N) | (%) | |
| TPS | 0.8 ± 0.2 | 2.8 ± 0.7 | 116.5 ± 30.5 |
| TPS + 5%NPC | 1.4 ± 0.4 | 4.9 ± 0.7 | 117.0 ± 25.0 |
| TPS + 10%NPC | 2.6 ± 0.5 | 6.0 ± 0.8 | 97.1 ± 29.0 |
| TPS + 15%NPC | 3.1 ± 0.9 | 5.7 ± 1.5 | 75.2 ± 10.0 |
| Sample | GTR (mol/m2s) | Permeability (mol/m2sPa) |
|---|---|---|
| 0% | 1.77 × 10−6 ± 3.25 × 10−7 | 1.74 × 10−11 ± 3.20 × 10−12 |
| 5% | 2.74 × 10−6 ± 1.32 × 10−6 | 2.70 × 10−11 ± 1.30 × 10−11 |
| 10% | 1.40 × 10−6 ± 2.25 × 10−6 | 1.39 × 10−11 ± 2.23 × 10−11 |
| 15% | 7.17 × 10−7 ± 6.13 × 10−7 | 2.59 × 10−11 ± 1.75 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palechor-Trochez, J.J.; Chantre-López, A.R.; Argote-Ortiz, E.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Effect of the Addition of Fique Bagasse Cellulose Nanoparticles on the Mechanical and Structural Properties of Plastic Flexible Films from Cassava Starch. Polymers 2023, 15, 4003. https://doi.org/10.3390/polym15194003
Palechor-Trochez JJ, Chantre-López AR, Argote-Ortiz E, Villada-Castillo HS, Solanilla-Duque JF. Effect of the Addition of Fique Bagasse Cellulose Nanoparticles on the Mechanical and Structural Properties of Plastic Flexible Films from Cassava Starch. Polymers. 2023; 15(19):4003. https://doi.org/10.3390/polym15194003
Chicago/Turabian StylePalechor-Trochez, Jhon Jairo, Adriana Rocio Chantre-López, Eduardo Argote-Ortiz, Héctor Samuel Villada-Castillo, and Jose Fernando Solanilla-Duque. 2023. "Effect of the Addition of Fique Bagasse Cellulose Nanoparticles on the Mechanical and Structural Properties of Plastic Flexible Films from Cassava Starch" Polymers 15, no. 19: 4003. https://doi.org/10.3390/polym15194003