Enhanced Water Sorption Performance of Polyacrylamide & Glass Fiber Paper Composites: Investigation and Comparison of Application in Desiccant Wheels
Abstract
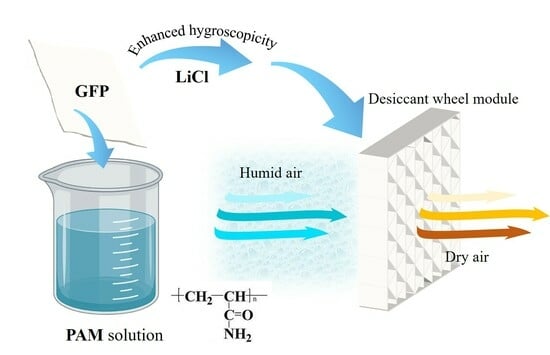
:1. Introduction
2. Experimental Materials and Method
2.1. Materials
2.2. Preparation of PAM
2.3. Preparation of Adsorbent&GFP
2.4. Characterization
2.5. Dehumidification and Desorption Performance Test
2.6. Uncertainty Analysis
2.7. Water Sorption Kinetic
3. Results and Discussion
3.1. Characterization of the Samples
3.1.1. FTIR Analysis
3.1.2. SEM and EDS Analysis
3.1.3. DSC Analysis
3.2. Water Sorption Performance Analysis
3.2.1. Optimization of PAM and LiCl Contents
3.2.2. Water Sorption Performance of Different Adsorbents & GFP
3.2.3. Dynamic Water Sorption Performance of Adsorbent&GFP
3.2.4. Water Sorption Kinetic Analysis
3.3. Desorption Performance Analysis
3.4. Multiple Dehumidification–Desorption Cycles
3.5. Performance Analysis of Desiccant Wheel Modules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| K | Rate coefficients, s−1 |
| ma | Initial mass of adsorbents, g |
| mb | GFP mass after loading PAM, g |
| mc | PAM&GFP mass after loading LiCl, g |
| me | Equilibrium mass of adsorbents, g |
| r | Desorption ratio, % |
| t | Time, s |
| w | Water uptake, g·g−1 |
| wa | Initial water uptake, g·g−1 |
| weq | Equilibrium water uptake, g·g−1 |
| wt | Dynamic water uptake, g·g−1 |
| R2 | Square of correlation coefficients |
References
- Tsuchida, S.; Umemura, H.; Murata, S.; Miyabe, A.; Satoh, M.; Matsushita, K.; Nakayama, T.; Nomura, F. Effect of humidity during sample preparation on bacterial identification using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Chromatogr. B 2021, 1176, 122780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zha, X.; Song, X.; Zhang, X. Optimization analysis of a hybrid fresh air handling system based on evaporative cooling and condensation dehumidification. Energy Convers. Manag. 2019, 180, 83–93. [Google Scholar] [CrossRef]
- Cho, H.-J.; Cheon, S.-Y.; Jeong, J.-W. Energy impact of vacuum-based membrane dehumidification in building air-conditioning applications. Appl. Therm. Eng. 2021, 182, 116094. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Zubair, S.M.; Abdallah, A.M.; Elbassoussi, M.H.; Ahmed, M.A. Novel and efficient integration of a humidification-dehumidification desalination system with an absorption refrigeration system. Appl. Energy 2020, 263, 114659. [Google Scholar] [CrossRef]
- Santosh, R.; Lee, H.-S.; Kim, Y.-D. A comprehensive review on humidifiers and dehumidifiers in solar and low-grade waste heat powered humidification-dehumidification desalination systems. J. Clean. Prod. 2022, 347, 131300. [Google Scholar] [CrossRef]
- Zhou, X.; Goldsworthy, M.; Sproul, A. Performance investigation of an internally cooled desiccant wheel. Appl. Energy 2018, 224, 382–397. [Google Scholar] [CrossRef]
- Intini, M.; Goldsworthy, M.; White, S.; Joppolo, C.M. Experimental analysis and numerical modelling of an AQSOA zeolite desiccant wheel. Appl. Therm. Eng. 2015, 80, 20–30. [Google Scholar] [CrossRef]
- Cheng, Q.; Pan, F.; Chen, B.; Jiang, Z. Preparation and dehumidification performance of composite membrane with PVA/gelatin–silica hybrid skin layer. J. Membr. Sci. 2010, 363, 316–325. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–432. [Google Scholar] [CrossRef]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, 9. [Google Scholar] [CrossRef]
- Solovyeva, M.V.; Gordeeva, L.G.; Krieger, T.A.; Aristov, Y.I. MOF-801 as a promising material for adsorption cooling: Equilibrium and dynamics of water adsorption. Energy Convers. Manag. 2018, 174, 356–363. [Google Scholar] [CrossRef]
- Youssef, P.G.; Dakkama, H.; Mahmoud, S.M.; Al-Dadah, R.K. Experimental investigation of adsorption water desalination/cooling system using CPO-27Ni MOF. Desalination 2017, 404, 192–199. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, B.; Huang, Y.; Qi, X.; Lou, F. Cooling capacity test for MIL-101 (Cr)/CaCl2 for adsorption refrigeration system. Molecules 2020, 25, 3975. [Google Scholar] [CrossRef] [PubMed]
- Mileo, P.G.M.; Cho, K.H.; Park, J.; Devautour-Vinot, S.; Chang, J.-S.; Maurin, G. Unraveling the water adsorption mechanism in the mesoporous MIL-100 (Fe) metal–organic framework. J. Phys. Chem. C 2019, 123, 23014–23025. [Google Scholar] [CrossRef]
- Zu, K.; Qin, M.; Cui, S. Progress and potential of metal-organic frameworks (MOFs) as novel desiccants for built environment control: A review. Renew. Sustain. Energy Rev. 2020, 133, 110246. [Google Scholar] [CrossRef]
- Ejeian, M.; Entezari, A.; Wang, R.Z. Solar powered atmospheric water harvesting with enhanced LiCl/MgSO4/ACF composite. Appl. Therm. Eng. 2020, 176, 115396. [Google Scholar] [CrossRef]
- Sun, Y.; Spieß, A.; Jansen, C.; Nuhnen, A.; Gökpinar, S.; Wiedey, R.; Ernst, S.-J.; Janiak, C. Tunable LiCl@ UiO-66 composites for water sorption-based heat transformation applications. J. Mater. Chem. A 2020, 8, 13364–13375. [Google Scholar] [CrossRef]
- An, H.; Chen, Y.; Wang, Y.; Liu, X.; Ren, Y.; Kang, Z.; Li, J.; Li, L. High-performance solar-driven water harvesting from air with a cheap and scalable hygroscopic salt modified metal–organic framework. Chem. Eng. J. 2023, 461, 141955. [Google Scholar] [CrossRef]
- Chen, L.; He, C. Experimental investigation of the dehumidification performance of a metal-organic framework MIL-101 (Cr)/ceramic fibre paper for use as a desiccant wheel. Microporous Mesoporous Mater. 2020, 305, 110378. [Google Scholar] [CrossRef]
- Mullangi, D.; Shalini, S.; Nandi, S.; Choksi, B.; Vaidhyanathan, R. Super-hydrophobic covalent organic frameworks for chemical resistant coatings and hydrophobic paper and textile composites. J. Mater. Chem. A 2017, 5, 8376–8384. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Hanikel, N.; Lyle, S.J.; Zhu, C.; Proserpio, D.M.; Yaghi, O.M. A Porous Covalent Organic Framework with Voided Square Grid Topology for Atmospheric Water Harvesting. J. Am. Chem. Soc. 2020, 142, 2218–2221. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Gropp, C.; Hanikel, N.; Möckel, A.; Lund, A.; Yaghi, O.M. Hydrazine-Hydrazide-Linked Covalent Organic Frameworks for Water Harvesting. ACS Cent. Sci. 2022, 8, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Aleid, S.; Wu, M.; Li, R.; Wang, W.; Zhang, C.; Zhang, L.; Wang, P. Salting-in effect of zwitterionic polymer hydrogel facilitates atmospheric water harvesting. ACS Mater. Lett. 2022, 4, 511–520. [Google Scholar] [CrossRef]
- Lei, C.; Guo, Y.; Guan, W.; Lu, H.; Shi, W.; Yu, G. Polyzwitterionic hydrogels for efficient atmospheric water harvesting. Angew. Chem. 2022, 134, e202200271. [Google Scholar] [CrossRef]
- Shi, Y.; Ilic, O.; Atwater, H.A.; Greer, J.R. All-day fresh water harvesting by microstructured hydrogel membranes. Nat. Commun. 2021, 12, 2797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, H.; Li, Z.; Huang, J.; Xu, Z.; Lai, Y.; Qian, X.; Zhang, S. Hydrogel materials for sustainable water resources harvesting & treatment: Synthesis, mechanism and applications. Chem. Eng. J. 2022, 439, 135756. [Google Scholar]
- Deng, F.; Chen, Z.; Wang, C.; Xiang, C.; Poredoš, P.; Wang, R. Hygroscopic Porous Polymer for Sorption-Based Atmospheric Water Harvesting. Adv. Sci. 2022, 9, 2204724. [Google Scholar] [CrossRef]
- Park, H.; Haechler, I.; Schnoering, G.; Ponte, M.D.; Schutzius, T.M.; Poulikakos, D. Enhanced atmospheric water harvesting with sunlight-activated sorption ratcheting. ACS Appl. Mater. Interfaces 2022, 14, 2237–2245. [Google Scholar] [CrossRef]
- Kim, S.; Liang, Y.; Kang, S.; Choi, H. Solar-assisted smart nanofibrous membranes for atmospheric water harvesting. Chem. Eng. J. 2021, 425, 131601. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Solid polymer desiccants based on poly(acrylic acid-co-acrylamide) and Laponite RD: Adsorption isotherm and kinetics studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124813. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super moisture-absorbent gels for all-weather atmospheric water harvesting. Adv. Mater. 2019, 31, 1806446. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, Y.; Alsaedi, M.; Wu, M.; Shi, L.; Wang, P. Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator. Environ. Sci. Technol. 2018, 52, 11367–11377. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Shi, W.; Zhang, J.H.; Chen, A.C.; Guan, W.X.; Lei, C.X.; Greer, J.R.; Boriskina, S.V.; Yu, G.H. Tailoring the Desorption Behavior of Hygroscopic Gels for Atmospheric Water Harvesting in Arid Climates. Adv. Mater. 2022, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Seo, S.W.; Mikšík, F.; Thu, K.; Miyazaki, T.; Ng, K.C. Effects of temperature and humidity ratio on the performance of desiccant dehumidification system under low-temperature regeneration. J. Therm. Anal. Calorim. 2023, 148, 3045–3058. [Google Scholar] [CrossRef]
- Meyer, P.W.; Cui, S.; Zeng, Y.; Aday, A.; Woods, J. Engineered Polymer Architectures for Thermo-Responsive Desiccants in Dehumidification Applications. Adv. Energy Mater. 2023, 2300990. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, C.; Han, J.; Zhao, Z.; Qi, X. Experimental evaluation of the dehumidification performance of a metal organic framework desiccant wheel. Int. J. Refrig. 2022, 133, 157–164. [Google Scholar] [CrossRef]
- Chung, J.Y.; Park, M.H.; Hong, S.H.; Baek, J.; Han, C.; Lee, S.; Kang, Y.T.; Kim, Y. Comparative performance evaluation of multi-objective optimized desiccant wheels coated with MIL-100 (Fe) and silica gel composite. Energy 2023, 283, 128567. [Google Scholar] [CrossRef]
- Bharathi, A.L.K.; Hussain, S.I.; Kalaiselvam, S. Performance evaluation of various fiber paper matrix desiccant wheels coated with nano-adsorbent for energy efficient dehumidification. Int. J. Refrig. 2023, 146, 416–429. [Google Scholar] [CrossRef]
- White, S.D.; Goldsworthy, M.; Reece, R.; Spillmann, T.; Gorur, A.; Lee, D.-Y. Characterization of desiccant wheels with alternative materials at low regeneration temperatures. Int. J. Refrig. 2011, 34, 1786–1791. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, C.-Y.; Chen, C.-C.; Chiang, Y.-C.; Chen, S.-L. Silica gel/polymer composite desiccant wheel combined with heat pump for air-conditioning systems. Energy 2016, 94, 87–99. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, C.; Han, J.; Qi, X.; Zhao, Z.; Teng, R. Dehumidification performance of aluminum fumarate metal organic framework and its composite. Appl. Therm. Eng. 2021, 199, 117570. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, X. Preparation and characterization of thermo-responsive composite for adsorption-based dehumidification and water harvesting. Chem. Eng. J. 2022, 429, 132498. [Google Scholar] [CrossRef]














| GFP + PAM (g) | +10% LiCl (g) | +15% LiCl (g) | +20% LiCl (g) | +25% LiCl (g) | +30% LiCl (g) | |
|---|---|---|---|---|---|---|
| GFP | 0.063 ± 0.0001 | / | / | / | / | / |
| GFP + 2%PAM | 0.0743 ± 0.0008 | 0.141 | 0.178 | 0.232 | 0.259 | 0.307 |
| GFP + 4%PAM | 0.0864 ± 0.0014 | 0.155 | 0.194 | 0.241 | 0.279 | 0.336 |
| GFP + 6%PAM | 0.1237 ± 0.0100 | 0.243 | 0.276 | 0.387 | 0.435 | 0.485 |
| RH | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | ||||||||||
| PAM&GFP | 0.00 | 0.00 | 0.01 | 0.02 | 0.05 | 0.09 | 0.14 | 0.19 | 0.31 | |
| AlFum-LiCl&GFP | 0.01 | 0.10 | 0.28 | 0.37 | 0.61 | 0.94 | 1.27 | 1.36 | 1.42 | |
| PAM-LiCl&GFP | 0.19 | 0.76 | 1.06 | 1.20 | 1.44 | 1.73 | 2.17 | 2.84 | 4.03 | |
| Parameters | 25 °C, 30%RH | 25 °C, 60%RH | |||
|---|---|---|---|---|---|
| Sample | K (s−1) | R2 | K (s−1) | R2 | |
| AlFum-LiCl&GFP | 10−4 | 0.990 | 10−4 | 0.991 | |
| PAM-LiCl&GFP | 10−4 | 0.995 | 10−4 | 0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Z.; Wang, Z.; Sun, W. Enhanced Water Sorption Performance of Polyacrylamide & Glass Fiber Paper Composites: Investigation and Comparison of Application in Desiccant Wheels. Polymers 2023, 15, 3678. https://doi.org/10.3390/polym15183678
Liu Y, Liu Z, Wang Z, Sun W. Enhanced Water Sorption Performance of Polyacrylamide & Glass Fiber Paper Composites: Investigation and Comparison of Application in Desiccant Wheels. Polymers. 2023; 15(18):3678. https://doi.org/10.3390/polym15183678
Chicago/Turabian StyleLiu, Yimo, Zhongbao Liu, Zepeng Wang, and Weiming Sun. 2023. "Enhanced Water Sorption Performance of Polyacrylamide & Glass Fiber Paper Composites: Investigation and Comparison of Application in Desiccant Wheels" Polymers 15, no. 18: 3678. https://doi.org/10.3390/polym15183678