Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
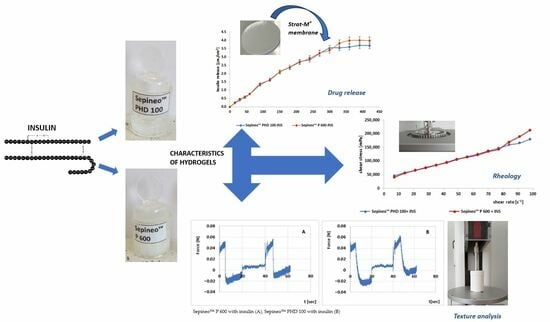
2.2. Preparation of Hydrogels
2.3. Stability Studies
2.4. Insulin Release In Vitro
2.5. Rheological Analysis
2.6. Texture Profile Analysis
2.7. Statistical Analysis
3. Results
3.1. Stability Studies
3.2. Insulin Release In Vitro
3.3. Rheological Analysis
3.4. Texture Profile Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ostróżka-Cieślik, A.; Przybyła, M.; Wójcik, W.; Birówka, K.; Majczyna, M.; Dolińska, B. Review of Research in Developing Hydrogels with Insulin to Promote Wound Healing. Med. Sci. Forum 2023, 21, 17. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X.; Wang, D.; Shi, Y.; Zhang, M.; Yu, T.; Liu, D.; Gao, M.; Zhang, X.; Liu, Y. Topical insulin application accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization. J. Cell Sci. 2020, 133, jcs235838. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J. Effects of Topical Insulin on Wound Healing: A Review of Animal and Human Evidences. Diabetes Metab. Syndr. Obes. 2020, 13, 719–727. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Liu, J.; Li, H. Insulin Topical Application for Wound Healing in Nondiabetic Patients. Comput. Math. Methods Med. 2021, 2021, 9785466. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhang, X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regen. 2012, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, T.; Liu, Y.; Chen, X.; Zhang, X. Topical Application of insulin accelerates vessel maturation of wounds by regulating angiopoietin-1 in diabetic mice. Int. J. Low. Extrem. Wounds 2015, 14, 353–364. [Google Scholar] [CrossRef]
- Dhall, S.; Silva, J.P.; Liu, Y.; Hrynyk, M.; Garcia, M.; Chan, A.; Lyubovitsky, J.; Neufeld, R.J.; Martins-Green, M. Release of insulin from PLGA-alginate dressing stimulates regenerative healing of burn wounds in rats. Clin. Sci. 2015, 129, 1115–1129. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. The Potential of Pharmaceutical Hydrogels in the Formulation of Topical Administration Hormone Drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Ioannovich, J.; Al-Amm, C.A.; El-Musa, K.A. Management of acute and chronic open wounds: The importance of moist environment in optimal wound healing. Curr. Pharm. Biotechnol. 2002, 3, 179–195. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, H.; An, S.; Jiang, Y. The relationship between oxygen permeability and phase separation morphology of the multicomponent silicone hydrogels. J. Phys. Chem. B 2014, 118, 14640–14647. [Google Scholar] [CrossRef] [PubMed]
- Madden, M.R.; Nolan, E.; Finkelstein, J.L.; Yurt, R.W.; Smeland, J.; Goodwin, C.W.; Hefton, J.; Staiano-Coico, L. Comparison of an Occlusive and a Semi-Occlusive Dressing and the Effect of the Wound Exudate upon Keratinocyte Proliferation. J. Trauma 1989, 29, 924–930. [Google Scholar] [CrossRef]
- Svensjo, T.; Pomahac, B.; Yao, F.; Slama, J.; Eriksson, E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast. Reconstr. Surg. 2000, 106, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Waśkiewicz, S.; Awietjan, S.; Wandzik, I. Thermoresponsive hydrogels with covalently incorporated trehalose as protein carriers. React. Funct. Polym. 2017, 119, 105–115. [Google Scholar] [CrossRef]
- Maggioni, D.; Cimicata, A.; Praticò, A.; Villa, R.; Bianchi, F.M.; Busoli Badiale, S.; Angelinetta, C. A Preliminary Clinical Evaluation of a Topical Product for Reducing Slight Rosacea Imperfections. Clin. Cosmet. Investig. Dermatol. 2020, 13, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Palmieri, G.F. Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS PharmSciTech 2009, 10, 368–375. [Google Scholar] [CrossRef]
- Khalil, E.A.; Majid, S.A.; Suaifan, G.A.; Al-Akayleh, F.T.; Sallam, A.S. Physicochemical characterization of emulgel formulated with SepineoP 600, SepineoSE 68 and cosolvent mixtures. Pharm. Dev. Technol. 2016, 21, 519–527. [Google Scholar] [CrossRef]
- Khan, B.A.; Ahmad, S.; Khan, M.K.; Hosny, K.M.; Bukhary, D.M.; Iqbal, H.; Murshid, S.S.; Halwani, A.A.; Alissa, M.; Menaa, F. Fabrication and Characterizations of Pharmaceutical Emulgel Co-Loaded with Naproxen-Eugenol for Improved Analgesic and Anti-Inflammatory Effects. Gels 2022, 8, 608. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, J.C. API-polymer interactions in Sepineo P600 based topical gel formulation-impact on rheology. Int. J. Pharm. 2022, 621, 121824. [Google Scholar] [CrossRef]
- Eudralex, G.M.P. The Rules Governing Medicinal Products in the European Union–Volume 4-EU Guidelines to Good Manufacturing Practice–Medicinal Products for Human and Veterinary Use–Annex 1–Manufacture of Sterile Medicinal Products; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Yoshida, R.; Kaneko, Y.; Sakai, K.; Okano, T.; Sakurai, Y.; Bae, Y.H. Positive thermosensitive pulsatile drug release using negative thermosensitive hydrogels. J. Control. Release 1994, 32, 97–102. [Google Scholar] [CrossRef]
- Quality of biotechnological products: Stability testing of biotechnological/biological products. Annex to the ICH Harmonised Tripartite Guideline for the Stability Testing of New Drug Substances and Products. Dev. Biol. Stand. 1998, 93, 211–219.
- U.S. Pharmacopeial Convention. United States Pharmacopeia, 32; U.S. Pharmacopeial Convention: Rockville, MD, USA, 2009; Volume 2, pp. 2282–2284. [Google Scholar]
- Cardia, M.C.; Carta, A.R.; Caboni, P.; Maccioni, A.M.; Erbì, S.; Boi, L.; Meloni, M.C.; Lai, F.; Sinico, C. Trimethyl Chitosan Hydrogel Nanoparticles for Progesterone Delivery in Neurodegenerative Disorders. Pharmaceutics 2019, 11, 657. [Google Scholar] [CrossRef]
- Mumuni, A.M.; Calister, E.U.; Aminu, N.; Franklin, C.K.; Musiliu Oluseun, A.; Usman, M.; Abdulmumuni, B.; James, Y.O.; Ofokansi, C.K.; Anthony, A.A.; et al. Mucin-Grafted Polyethylene Glycol Microparticles Enable Oral Insulin Delivery for Improving Diabetic Treatment. Appl. Sci. 2020, 10, 2649. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fónagy, K.; Bors, L.A.; Iván, K.; Erdő, F. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Brewster, D.; Colonero, P. In vitro diffusion studies in transdermal research: A synthetic membrane model in place of human skin. Drug Dev. Deliv. 2012, 12, 40–42. [Google Scholar]
- Kaur, L.; Singh, K.; Paul, S.; Singh, S.; Singh, S.; Jain, S.K. A Mechanistic Study to Determine the Structural Similarities Between Artificial Membrane Strat-M™ and Biological Membranes and Its Application to Carry Out Skin Permeation Study of Amphotericin B Nanoformulations. AAPS PharmSciTech 2018, 19, 1606–1624. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M(R) synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef]
- Milanowski, B.; Wosicka-Frąckowiak, H.; Główka, E.; Sosnowska, M.; Woźny, S.; Stachowiak, F.; Suchenek, A.; Wilkowski, D. Optimization and Evaluation of the In Vitro Permeation Parameters of Topical Products with Non-Steroidal Anti-Inflammatory Drugs through Strat-M® Membrane. Pharmaceutics 2021, 13, 1305. [Google Scholar] [CrossRef]
- Arce, F.J.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of Artificial Membrane, Strat-M®, in the Assessment of Drug Permeation from Complex Vehicles in Finite Dose Conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity and In Vivo Evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef]
- EMA. Guideline on the Investigation of Bioequivalence; (CPMP/EWP/QWP/1401/98 Rev. 1/ Corr **); EMA: London, UK, 2010. [Google Scholar]
- FDA. Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs—General Considerations; Food and Drug Administration Guidance for Industry; Food and Drug Administration: Rockville, MD, USA, 2014. [Google Scholar]
- Alatzoglou, F.-E.G.; Vassaki, M.; Nirgianaki, K.; Tripodianos, E.; Turhanen, P.; Demadis, K.D.; Papathanasiou, K.E. Surface-Modified Silica Hydrogels for the Programmable Release of Bisphosphonate Anti-Osteoporosis Drugs: The Case of Etidronate. Materials 2023, 16, 3379. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical and Physiochemical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Bruschi, M.L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Pereira, E.D.; da Silva Dutra, L.; Paiva, T.F.; de Almeida Carvalho, L.L.; Rocha, H.V.A.; Pinto, J.C. In Vitro Release and In Vivo Pharmacokinetics of Praziquantel Loaded in Different Polymer Particles. Materials 2023, 16, 3382. [Google Scholar] [CrossRef]
- Ghosal, K.; Ray, S.D. Alginate/hydrophobic HPMC (60M) particulate systems: New matrix for site-specific and controlled drug delivery. Braz. J. Pharm. Sci. 2011, 47, 833–844. [Google Scholar] [CrossRef]
- Shah, H.P. Quality by design enabled development and optimization of gastroretentive floating matrix tablets of dipyridamole. Asian J. Pharm. 2017, 11. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and Hemocompatible pH-Responsive Hydrogel for Skin Wound Healing Application: In Vitro Drug Release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Pollice, A.; Giordano, C.; Laera, G.; Saturno, D.; Mininni, G. Rheology of sludge in a complete retention membrane bioreactor. Environ. Technol. 2006, 27, 723–732. [Google Scholar] [CrossRef]
- Ozcan, I.; Abaci, O.; Uztan, A.H.; Aksu, B.; Boyacioglu, H.; Guneri, T.; Ozer, O. Enhanced topical delivery of terbinafine hydrochloride with chitosan hydrogels. AAPS PharmSciTech 2009, 10, 1024–1031. [Google Scholar] [CrossRef]
- Yoo, D.; Yoo, B. Rheology of Rice Starch-Sucrose Composites. Starch-Stärke 2005, 57, 254–261. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Chiarentin, L.; Cardoso, C.; Miranda, M.; Vitorino, C. Rheology of Complex Topical Formulations: An Analytical Quality by Design Approach to Method Optimization and Validation. Pharmaceutics 2023, 15, 1810. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.; Orlu, M.; Araman, A. Evaluation of Mechanical and Mucoadhesive Properties of Clomiphene Citrate Gel Formulations Containing Carbomers and Their Thiolated Derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Tan, Y.T.; Peh, K.K.; Hanbali, A.O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech 2000, 1, 69–78. [Google Scholar] [CrossRef]
- Suarez, D.M.; Manca, E.; Crupkin, M.; Paredi, M.E. Emulsifying and gelling properties of weakfish myofibrillar proteins as affected by squid mantle myofibrillar proteins in a model system. Braz. J. Food Technol. 2014, 17, 8–18. [Google Scholar] [CrossRef]
- Pérez-Calixto, D.; Amat-Shapiro, S.; Zamarrón-Hernández, D.; Vázquez-Victorio, G.; Puech, P.-H.; Hautefeuille, M. Determination by Relaxation Tests of the Mechanical Properties of Soft Polyacrylamide Gels Made for Mechanobiology Studies. Polymers 2021, 13, 629. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Quitério, M.; Simões, S.; Ascenso, A.; Carvalheiro, M.; Leandro, A.P.; Correia, I.; Viana, A.S.; Faísca, P.; Ascensão, L.; Molpeceres, J.; et al. Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. Int. J. Mol. Sci. 2021, 22, 4087. [Google Scholar] [CrossRef]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef]
- Pünnel, L.C.; Lunter, D.J. Film-Forming Systems for Dermal Drug Delivery. Pharmaceutics 2021, 13, 932. [Google Scholar] [CrossRef]
- Alberti, I.; Grenier, A.; Kraus, H.; Carrara, D.N. Pharmaceutical development and clinical effectiveness of a novel gel technology for transdermal drug delivery. Expert Opin. Drug Deliv. 2005, 2, 935–950. [Google Scholar] [CrossRef]
- Coussot, P. Yield stress fluid flows: A review of experimental data. J. Non-Newton. Fluid Mech. 2014, 211, 31–49. [Google Scholar] [CrossRef]
- Quemada, D.; Berli, C. Describing the Flow Curve of Shear-Banding Fluids Through a Structural Minimal Model. arXiv 2009, arXiv:0903.0808. [Google Scholar]
- Von Zuben, E.d.S.; Eloy, J.O.; Inácio, M.D.; Araujo, V.H.S.; Baviera, A.M.; Gremião, M.P.D.; Chorilli, M. Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics 2022, 14, 2492. [Google Scholar] [CrossRef]
- Agrawal, A.; Gupta, P.; Khanna, A.; Sharma, R.; Chandrabanshi, H.; Gupta, N.; Patil, U.; Yadav, S. Development and characterization of in situ gel system for nasal insulin delivery. Die Pharm.-Int. J. Pharm. Sci 2010, 65, 188–193. [Google Scholar]
- Tasdighi, E.; Azar, Z.J.; Mortazavi, S.A. Development and In-vitro evaluation of a contraceptive vagino-adhesive propranolol hydrochloride gel. Iran J. Pharm. Res. 2012, 11, 13–26. [Google Scholar]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Pluta, J.; Karolewicz, B. Hydrogels: Properties and application in the technology of drug form. I. The characteristic hydrogels. Polim Med. 2004, 34, 3–19. [Google Scholar]
- Qu, X.; Wirsén, A.; Albertsson, A. Novel Ph-sensitive chitosan hydrogels: Swelling behavior and states of water. Polymer 2000, 41, 4589–4598. [Google Scholar] [CrossRef]
- Cai, Y.; Che, J.; Yuan, M.; Shi, X.; Chen, W.; Yuan, W.E. Effect of glycerol on sustained insulin release from PVA hydrogels and its application in diabetes therapy. Exp. Ther. Med. 2016, 12, 2039–2044. [Google Scholar] [CrossRef]
- Sadagurski, M.; Nofech-Mozes, S.; Weingarten, G.; White, M.F.; Kadowaki, T.; Wertheimer, E. Insulin receptor substrate 1 (IRS-1) plays a unique role in normal epidermal physiology. J. Cell. Physiol. 2007, 213, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Guildford, L.; Crofts, C.; Lu, J. Can the Molar Insulin: C-Peptide Ratio Be Used to Predict Hyperinsulinaemia? Biomedicines 2020, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, F.; Kan, J.; Deng, J.; Wang, B.; Hao, S. Synthesis and fabrication of a keratin-conjugated insulin hydrogel for the enhancement of wound healing. Colloids Surf. B Biointerfaces 2019, 175, 436–444. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szucs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Nallagundla, S.; Patnala, S.; Kanfer, I. Comparison ofin vitro release rates of acyclovir from cream formulations using vertical diffusion cells. AAPS Pharm. Sci. Tech. 2014, 15, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of Formulation Parameters on Permeation of Ibuprofen from Topical Formulations Using Strat-M® Membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- CHMP. Draft Guideline on Quality and Equivalence of Topical Products; European Medicines Agency: Amsterdam, The Netherlands, 2018; pp. 1–36. Available online: https://www.ema.europa.eu/en/documents/scientificguideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 21 July 2023).
- Li, J.; Lee, I.; Shin, G.H.; Chen, X.; Park, H.J. Curcumin-Eudragit®EPO solid dispersion: A simple and potent method to solve the problems of curcumin. Eur. J. Pharm. Biopharm. 2015, 94, 322–332. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater. Sci. Eng. C 2017, 72, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Vaez, A.; Samadian, H.; Sahrapeyma, H.; Mirzaii, M.; Ghorbani, S.; Goodarzi, A. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int. J. Biol. Macromol. 2018, 117, 601–609. [Google Scholar] [CrossRef]




| Sepineo™ P 600 Hydrogel G1 [%w/w] | Sepineo™ PHD 100 Hydrogel G2 [%w/w] | |
|---|---|---|
| Sepineo™ P 600 | 4 | - |
| Sepineo™ PHD | - | 2 |
| Glycerol 85% | 3 | 3 |
| Distilled water | Ad 100 | Ad 100 |
| Conditions | Weeks | Color | Phase Separation | pH | Drug Content | Viscosity η (30 s−1) [mPa × s] | Photos of Hydrogels |
|---|---|---|---|---|---|---|---|
| G1-INS | |||||||
| 5 ± 3 °C | 2 | No color change | No | 7.11 ± 0.05 | 99.5% ± 0.5 | 3940 ± 204 |  |
| 4 | No color change | No | 7.12 ± 0.03 | 99.6% ± 0.5 | 3960 ± 114 |  | |
| 25 ± 1 °C | 2 | No color change | No | 7.16 ± 0.05 | 99.7% ± 0.4 | 4080 ± 199 |  |
| 4 | No color change | No | 7.15 ± 0.02 | 100.5% ± 0.2 | 4091 ± 200 |  | |
| G2-INS | |||||||
| 5 ± 3 °C | 2 | No color change | No | 7.16 ± 0.04 | 99.2% ± 0.4 | 4320 ± 180 |  |
| 4 | No color change | No | 7.11 ± 0.03 | 99.8% ± 0.3 | 4510 ± 211 |  | |
| 25 ± 1 °C | 2 | No color change | No | 7.09 ± 0.04 | 99.8% ± 0.2 | 4680 ± 202 |  |
| 4 | No color change | No | 7.11 ± 0.01 | 101.2% ± 0.5 | 4820 ± 200 |  | |
| Kinetics Models | Equation | Hydrogel | Parameters | R2adjusted | AIC | MSC |
|---|---|---|---|---|---|---|
| Zero Order model | f = k0 × t | G1-INS G2-INS | k0 = 0.012 k0 = 0.011 | 0.9790 0.9369 | −9.0372 7.1041 | 3.7198 2.6294 |
| First Order model | f = 100 × [1 − e−k1×t] | G1-INS G2-INS | k1 = 0.001 k1 = 0.001 | 0.9813 0.9412 | −10.6466 6.0386 | 3.8347 2.7004 |
| Higuchi model | f = kH × t0.5 | G1-INS G2-INS | kH = 0.184 kH = 0.181 | 0.8933 0.9123 | 13.7068 12.0342 | 2.0952 2.3007 |
| Korsmeyer-Peppas model | f = kKP × tn | G1-INS | kKP = 0.030 | 0.9946 | −27.2065 | 5.0176 |
| n = 0.834 | ||||||
| G2-INS | kKP = 0.046 | |||||
| n = 0.749 | 0.9796 | −8.9155 | 3.6973 | |||
| Peppas-Sahlin model | f = kPS1 × tm + kPS2 × t(2×m) | G1-INS | kPS1 = −0.220 | 0.9961 | −30.7982 | 5.2741 |
| kPS2 =0.121 | ||||||
| m = 0.324 | ||||||
| G2-INS | kPS1 = −0.973 | 0.9882 | −16.3418 | 4.1924 | ||
| kPS2 = 0.561 | ||||||
| m = 0.217 | ||||||
| Hixson-Crowell model | f = 100 × [1 − (1 − kHC × t)3] | G1-INS G2-INS | kHC = 0.0 kHC = 0.0 | 0.9805 0.9398 | −10.1033 6.3962 | 3.7959 2.6766 |
| Hopfenberg model | f = 100 × [1 − (1 − kHB × t)n] | G1-INS | kHB = 0.0 | 0.9797 | −8.6180 | 3.6869 |
| n = 58.345 | ||||||
| G2-INS | kHB = 0.0 | 0.9366 | 8.0568 | 2.5659 | ||
| n = 58.939 | ||||||
| Baker–Lonsdale model | 3/2 × [1−(1 − F/100)2/3] − F/100 = kBL × t | G1-INS G2-INS | kBL = 0.0 kBL = 0.0 | 0.8915 0.9108 | 13.9423 12.2931 | 2.0784 2.2834 |
| Formula Code | Herschel–Bulkley | Ostwald-de Waele | Bingham | Casson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| τ0 | n | K | R2 | n | K | R2 | τ0 | R2 | τ0 | R2 | |
| G1-INS | 32200 | 0.993 | 2.84 | 0.912 | 0.930 | 3.76 | 0.760 | 74282 | 0.639 | 4701 | 0.688 |
| G2-INS | 48000 | 0.945 | 3.30 | 0.983 | 0.538 | 15.5 | 0.901 | 33006 | 0.880 | 15712 | 0.905 |
| Hydrogel | η (30 s−1) [mPa × s] | η (50 s−1) [mPa × s] | η (100 s−1) [mPa × s] |
|---|---|---|---|
| G1-INS | 3241 ± 50 | 1949 ± 91 | 1387 ± 71 |
| G2-INS | 3772 ± 87 | 2070 ± 101 | 1462 ± 113 |
| Formula Code | Relaxation [%] | Hardness 1 [N] | Hardness 2 [N] | Cohesiveness | Adhesiveness [mJ] | Elasticity |
|---|---|---|---|---|---|---|
| G1-INS | 76.4% ± 1.06 | 0.053 ± 0.01 | 0.057 ± 0.03 | 1.579 ± 0.05 | 0.2 ± 0.02 | 0.693 ± 0.07 |
| G2-INS | 71.5% ± 0.62 | 0.055 ± 0.02 | 0.064 ± 0.01 | 1.671 ± 0.06 | 0.3 ± 0.01 | 0.716 ± 0.05 |
| p | <0.05 | NS | <0.05 | <0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Wilczyński, S.; Dolińska, B. Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane. Polymers 2023, 15, 3639. https://doi.org/10.3390/polym15173639
Ostróżka-Cieślik A, Wilczyński S, Dolińska B. Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane. Polymers. 2023; 15(17):3639. https://doi.org/10.3390/polym15173639
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Sławomir Wilczyński, and Barbara Dolińska. 2023. "Hydrogel Formulations for Topical Insulin Application: Preparation, Characterization and In Vitro Permeation across the Strat-M® Membrane" Polymers 15, no. 17: 3639. https://doi.org/10.3390/polym15173639