Polypyrrole- and Polyaniline-Coated Cotton Fabrics as Efficient Adsorbents for the Pharmaceutical Water Contaminants Diclofenac and Salicylic Acid
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of PPY- and PANI-Coated Cotton Fabrics
2.3. Characterization
2.4. Adsorption and Regeneration Studies
2.5. Modeling Investigation
3. Results and Discussion
3.1. Characterization of PPY-Coated and PANI-Coated Cotton Fabrics
3.2. Adsorption Studies
3.2.1. Effect of pH
3.2.2. Time Profiles and Kinetics
3.2.3. Adsorption Isotherms
3.2.4. Parameters Affecting the Adsorption Process
4. Regeneration
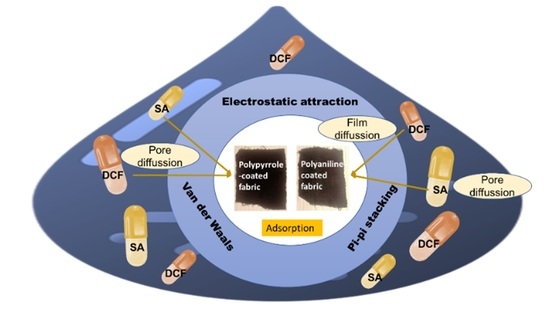
5. Adsorption Mechanism
6. Comparison with Literature
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, K.N.; Rehman, M.S.U.; Mustafa, G.; Nazar, M.F.; Sun, Q.; Iqbal, J.; Mulla, S.I.; Yu, C.-P. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol. Environ. Saf. 2017, 136, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavinia, F.; Abdolmaleki, M.; Amiri, M.; Khalafi-Nezhad, A. Removal of salicylic acid as an emerging contaminant by a polar nano-dendritic adsorbent from aqueous media. J. Colloid Interface Sci. 2017, 493, 138–149. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.; Pretti, C.; Faggio, C. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to salicylic acid. Aquat. Toxicol. 2019, 214, 105258. [Google Scholar] [CrossRef]
- Silva, M.; Baltrus, J.; Williams, C.; Knopf, A.; Zhang, L.; Baltrusaitis, J. Mesoporous Fe-doped MgO nanoparticles as a heterogeneous photo-Fenton-like catalyst for degradation of salicylic acid in wastewater. J. Environ. Chem. Eng. 2021, 9, 105589. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.R.; Oliveira, M.F.; da Silva, M.G.; Vieira, M.G. Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: A review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Natarajan, R.; Saikia, K.; Ponnusamy, S.K.; Rathankumar, A.K.; Rajendran, D.S.; Venkataraman, S.; Tannani, D.B.; Arvind, V.; Somanna, T.; Banerjee, K. Understanding the factors affecting adsorption of pharmaceuticals on different adsorbents–A critical literature update. Chemosphere 2022, 287, 131958. [Google Scholar] [CrossRef]
- Farghal, H.H.; Nebsen, M.; El-Sayed, M.M. Multifunctional Chitosan/Xylan-Coated Magnetite Nanoparticles for the Simultaneous Adsorption of the Emerging Contaminants Pb (II), Salicylic Acid, and Congo Red Dye. Water 2023, 15, 829. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, V.T.; Garina, E.O.; Burtseva, E.I.; Kirillova, E.S.; Ivanova, M.V.; Stejskal, J.; Sapurina, I.Y. Conducting polymers as sorbents of influenza viruses. Chem. Pap. 2017, 71, 495–503. [Google Scholar] [CrossRef]
- Amer, W.A.; Omran, M.M.; Rehab, A.F.; Ayad, M.M. Acid green crystal-based in situ synthesis of polyaniline hollow nanotubes for the adsorption of anionic and cationic dyes. RSC Adv. 2018, 8, 22536–22545. [Google Scholar] [CrossRef] [PubMed]
- Fraga, V.M.; Lovi, I.T.; Abegão, L.M.; Mello, H.J. Understanding the Effect of Deposition Technique on the Structure–Property Relationship of Polyaniline Thin Films Applied in Potentiometric pH Sensor. Polymers 2023, 15, 3450. [Google Scholar] [CrossRef]
- Zaghlol, S.; Amer, W.A.; Shaaban, M.H.; Ayad, M.M.; Bober, P.; Stejskal, J. Conducting macroporous polyaniline/poly (vinyl alcohol) aerogels for the removal of chromium (VI) from aqueous media. Chem. Pap. 2020, 74, 3183–3193. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Zaghlol, S.; Maráková, N.; Stejskal, J. Polypyrrole-coated cotton fabric decorated with silver nanoparticles for the catalytic removal of p-nitrophenol from water. Cellulose 2018, 25, 7393–7407. [Google Scholar]
- Ayad, M.M.; Amer, W.A.; Zaghlol, S.; Minisy, I.M.; Bober, P.; Stejskal, J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018, 72, 1605–1618. [Google Scholar] [CrossRef]
- Ghorbani, M.; Esfandian, H.; Taghipour, N.; Katal, R. Application of polyaniline and polypyrrole composites for paper mill wastewater treatment. Desalination 2010, 263, 279–284. [Google Scholar]
- Li, S.; Qian, K.; Wang, S.; Liang, K.; Yan, W. Polypyrrole-grafted coconut shell biological carbon as a potential adsorbent for methyl tert-butyl ether removal: Characterization and adsorption capability. Int. J. Environ. Res. Public Health 2017, 14, 113. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb (II) and Cd (II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Minella, M.; Fabbri, D.; Calza, P.; Minero, C. Selected hybrid photocatalytic materials for the removal of drugs from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 11–17. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Zhou, M.; Heinze, J. Electropolymerization of pyrrole and electrochemical study of polypyrrole. 2. Influence of acidity on the formation of polypyrrole and the multipathway mechanism. J. Phys. Chem. B 1999, 103, 8443–8450. [Google Scholar] [CrossRef]
- Prabhu, R.; Shetty, K.; Jeevananda, T.; Murthy, H.A.; Sillanpaa, M.; Nhat, T. Novel polyaniline–halloysite nanoclay hybrid composites: Synthesis, physico-chemical, thermal and electrical properties. Inorg. Chem. Commun. 2023, 148, 110328. [Google Scholar] [CrossRef]
- Feng, X.; Patterson, D.A.; Balaban, M.; Emanuelsson, E.A.C. Enabling the utilization of wool as an enzyme support: Enhancing the activity and stability of lipase immobilized onto woolen cloth. Colloids Surf. B Biointerfaces 2013, 102, 526–533. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S. Salt-free reactive dyeing of the cotton fabric modified with chitosan-poly (propylene imine) dendrimer hybrid. Fibers Polym. 2015, 16, 1075–1081. [Google Scholar] [CrossRef]
- Madkour, T.M.; Barakat, A.M. Computer simulation of polymers. Comput. Theor. Polym. Sci. 1997, 7, 35–46. [Google Scholar] [CrossRef]
- Hou, G.; Ren, R.; Shang, W.; Weng, Y.; Liu, J. Molecular Dynamics Simulation of Polymer Nanocomposites with Supramolecular Network Constructed via Functionalized Polymer End-Grafted Nanoparticles. Polymers 2023, 15, 3259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Q.; Gao, X.; Piao, G. A nanocellulose polypyrrole composite based on tunicate cellulose. Int. J. Polym. Sci. 2013, 2013, 175609. [Google Scholar] [CrossRef]
- Al-Aoh, H.A.; Badi, N.; Roy, A.S.; Alsharari, A.M.; Abd El Wanees, S.; Albaqami, A.; Ignatiev, A. Preparation of Anionic Surfactant-Based One-Dimensional Nanostructured Polyaniline Fibers for Hydrogen Storage Applications. Polymers 2023, 15, 1658. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline–TiO2 composite and its comparative corrosion protection performance with polyaniline. Synth. Met. 2007, 157, 205–213. [Google Scholar] [CrossRef]
- Šeděnková, I.; Trchová, M.; Stejskal, J. Thermal degradation of polyaniline films prepared in solutions of strong and weak acids and in water–FTIR and Raman spectroscopic studies. Polym. Degrad. Stab. 2008, 93, 2147–2157. [Google Scholar] [CrossRef]
- Thao, V.D.; Giang, B.L.; Thu, T.V. Free-standing polypyrrole/polyaniline composite film fabricated by interfacial polymerization at the vapor/liquid interface for enhanced hexavalent chromium adsorption. RSC Adv. 2019, 9, 5445–5452. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar]
- Pandey, P.; Sharma, S.; Sambi, S. Kinetics and equilibrium study of chromium adsorption on zeoliteNaX. Int. J. Environ. Sci. Technol. 2010, 7, 395–404. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.-C.; Zhang, Q.-J.; Zhang, W.-M.; Zhang, Q.-X. Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Pires, B.C.; do Nascimento, T.A.; Dutra, F.V.A.; Borges, K.B. Removal of a non-steroidal anti-inflammatory by adsorption on polypyrrole/multiwalled carbon nanotube composite—Study of kinetics and equilibrium in aqueous medium. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123583. [Google Scholar] [CrossRef]
- Bajpai, S.; Bhowmik, M. Adsorption of diclofenac sodium from aqueous solution using polyaniline as a potential sorbent. I. Kinetic studies. J. Appl. Polym. Sci. 2010, 117, 3615–3622. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Xiao, G.; Wen, R.; Liu, A.; He, G.; Wu, D. Adsorption performance of salicylic acid on a novel resin with distinctive double pore structure. J. Hazard. Mater. 2017, 329, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, H.; Xu, W.; Zhou, W.; Zhou, Z.; Yang, W. Selective adsorption of dibenzothiophene using magnetic molecularly imprinted polymers. Adsorpt. Sci. Technol. 2012, 30, 331–343. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, Y.Q.; Zhu, H.Y.; Yao, J.; Xiao, L. Removal of methyl orange from aqueous solutions by magnetic maghemite/chitosan nanocomposite films: Adsorption kinetics and equilibrium. J. Appl. Polym. Sci. 2012, 125, E540–E549. [Google Scholar] [CrossRef]
- Hussain, S.; Kamran, M.; Khan, S.A.; Shaheen, K.; Shah, Z.; Suo, H.; Khan, Q.; Shah, A.B.; Rehman, W.U.; Al-Ghamdi, Y.O. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 2021, 168, 383–394. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Kandil, H.; Abdelhamid, A.E.; Moghazy, R.M.; Amin, A. Functionalized PVA film with good adsorption capacity for anionic dye. Polym. Eng. Sci. 2022, 62, 145–159. [Google Scholar] [CrossRef]
- Jawad, A.H.; Islam, M.A.; Hameed, B. Cross-linked chitosan thin film coated onto glass plate as an effective adsorbent for adsorption of reactive orange 16. Int. J. Biol. Macromol. 2017, 95, 743–749. [Google Scholar] [CrossRef]
- Akbal, F. Adsorption of basic dyes from aqueous solution onto pumice powder. J. Colloid Interface Sci. 2005, 286, 455–458. [Google Scholar] [CrossRef]
- Javadian, H.; Angaji, M.T.; Naushad, M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes. J. Ind. Eng. Chem. 2014, 20, 3890–3900. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, P.; Zhang, H.; Li, X.; He, Y.; Qin, P.; Yang, C. Tough porous nanocomposite hydrogel for water treatment. J. Hazard. Mater. 2022, 421, 126754. [Google Scholar] [CrossRef]
- Feng, J.-B.; Li, Y.-Y.; Zhang, Y.; Xu, Y.-Y.; Cheng, X.-W. Adsorptive removal of indomethacin and diclofenac from water by polypyrrole doped-GO/COF-300 nanocomposites. Chem. Eng. J. 2022, 429, 132499. [Google Scholar] [CrossRef]
- Higgins, P.; Siddiqui, S.H. Efficacy of Polyaniline (PANI) nanofibres for capturing Diclofenac (DC) drug from its aqueous solutions. J. Indian Chem. Soc. 2022, 99, 100494. [Google Scholar] [CrossRef]
- Ravi, S.; Choi, Y.; Choe, J.K. Novel phenyl-phosphate-based porous organic polymers for removal of pharmaceutical contaminants in water. Chem. Eng. J. 2020, 379, 122290. [Google Scholar] [CrossRef]
- Shaipulizan, N.S.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Kamaruzaman, S.; Subri, N.N.S.; Othman, N. Hypercrosslinked poly (AN-co-EGDMA-co-VBC): Synthesis via suspension polymerization, characterizations, and potential to adsorb diclofenac and metformin from aqueous solution. Colloid Polym. Sci. 2020, 298, 1649–1667. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Wang, J.-X. Preparation of phosphorus based hyper cross-linked polymers and adsorption of salicylic acid from aqueous solution. J. Mol. Struct. 2020, 1221, 128804. [Google Scholar] [CrossRef]











| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||||
|---|---|---|---|---|---|---|---|---|
| qe, mg g−1 | k1, min−1 | R2 | RMSE | qe, mg g−1 | k2, g mg−1 min−1 | R2 | RMSE | |
| DCF-PPY | 4.86 | 0.027 | 0.8336 | 0.229 | 30.03 | 0.01270 | 0.9998 | 0.035 |
| SA-PPY | 15.02 | 0.0207 | 0.9321 | 0.192 | 23.92 | 0.00021 | 0.8475 | 1.449 |
| DCF-PANI | 2.62 | 0.0094 | 0.9011 | 0.045 | 19.08 | 0.02738 | 0.9998 | 0.021 |
| SA-PANI | 3.76 | 0.0117 | 0.8321 | 1.822 | 14.64 | 0.01577 | 0.9977 | 0.194 |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| qm, mg/g | Kd, L/mg | R2 | RMSE | 1/n | Kf, (mg/g) × (L/mg)n | R2 | RMSE | |
| DCF-PPY | 136.99 | 5.00 | 0.8314 | 3.20 | 0.5836 | 30.52 | 0.9212 | 3.17 |
| DCF-PANI | 26.25 | 10.51 | 0.9899 | 0.86 | 0.4544 | 4.05 | 0.8942 | 1.78 |
| SA-PPY | 21.01 | 8.649 | 0.9930 | 0.66 | 0.4114 | 3.92 | 0.9666 | 1.06 |
| SA-PANI | 16.07 | 17.27 | 0.8815 | 1.09 | 0.3966 | 2.49 | 0.9404 | 0.76 |
| CED (J cm−3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| System | Total | Van Der Waals | Electrostatic | ||||||
| Ratio | 30/70 | 50/50 | 70/30 | 30/70 | 50/50 | 70/30 | 30/70 | 50/50 | 70/30 |
| PPY/DCF | 344.8 | 345.4 | 338.5 | 270.2 | 283.4 | 290.5 | 60.26 | 53.35 | 40.38 |
| PANI/DCF | 313.9 | 330.6 | 346.3 | 218 | 272.7 | 296.9 | 53.91 | 50.51 | 41.29 |
| PPY/SA | 518.4 | 461.2 | 423.5 | 291.2 | 299.9 | 309 | 217.9 | 154.7 | 106.8 |
| PANI/SA | 488.2 | 457.5 | 415.4 | 283.2 | 294.2 | 304.8 | 204.1 | 155.8 | 102.8 |
| Adsorbent | Contaminant | qm, (mg/g) | Reference |
|---|---|---|---|
| Phenyl-phosphate-based porous organic polymers | DCF | 217 (Langmuir) | [54] |
| Chitosan/xylan-coated magnetite nanoparticles | SA | 11.34 (Freundlich) | [10] |
| Poly(acrylonitrile-co-ethylene glycol dimethacrylate-co-vinylbenzyl chloride) | DCF | 61 (Langmuir) | [55] |
| Phosphorus based hyper cross-linked polymers | SA | 369 (Langmuir) | [56] |
| PPY-coated fabrics | DCF | 65.61 (Freundlich) | This work |
| SA | 21.01 (Langmuir) | ||
| PANI-coated fabrics | DCF | 26.25 (Langmuir) | This work |
| SA | 12.92 (Freundlich) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farghal, H.H.; Tawakey, S.H.; Amer, W.A.; Ayad, M.M.; Madkour, T.M.; El-Sayed, M.M.H. Polypyrrole- and Polyaniline-Coated Cotton Fabrics as Efficient Adsorbents for the Pharmaceutical Water Contaminants Diclofenac and Salicylic Acid. Polymers 2023, 15, 3563. https://doi.org/10.3390/polym15173563
Farghal HH, Tawakey SH, Amer WA, Ayad MM, Madkour TM, El-Sayed MMH. Polypyrrole- and Polyaniline-Coated Cotton Fabrics as Efficient Adsorbents for the Pharmaceutical Water Contaminants Diclofenac and Salicylic Acid. Polymers. 2023; 15(17):3563. https://doi.org/10.3390/polym15173563
Chicago/Turabian StyleFarghal, Hebatullah H., Samar H. Tawakey, Wael A. Amer, Mohamad M. Ayad, Tarek M. Madkour, and Mayyada M. H. El-Sayed. 2023. "Polypyrrole- and Polyaniline-Coated Cotton Fabrics as Efficient Adsorbents for the Pharmaceutical Water Contaminants Diclofenac and Salicylic Acid" Polymers 15, no. 17: 3563. https://doi.org/10.3390/polym15173563