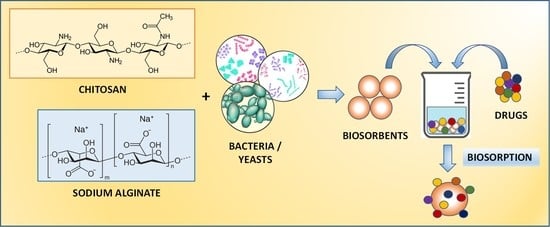
Insights into Recent Advances of Biomaterials Based on Microbial Biomass and Natural Polymers for Sustainable Removal of Pharmaceuticals Residues
Abstract
:1. Introduction
2. Bibliographic Research Methodology
3. Biosorption—Concept and Current Perspectives
4. Biosorbents—Types and Applications in the Removal of Pharmaceutical Residues
4.1. Microorganisms and Residual Microbial Biomass
| Biosorbent | Therapeutic Group/ Pharmaceutical Compound | Process Parameters | Obtained Results | Ref. |
|---|---|---|---|---|
| BACTERIAL BIOMASS | ||||
| Antibiotics | ||||
| Bacillus subtilis 1156WTNCC strain (active cells) | Amoxicillin | pH = 6.5; temperature = 35 °C; time = 12 days; initial concentration of pharmaceutical compound = 0.2 ÷ 5.0 mg/mL; aerobic conditions; batch system | Maximum biodegradation efficiency 25.03% for C0 = 1 mg/mL | [68] |
| Ampicillin | Maximum biodegradation efficiency 15.59% for C0 = 0.8 mg/mL | |||
| Cephalexin | Maximum biodegradation efficiency 22.59% for C0 = 1.0 mg/mL | |||
| Cefuroxime | Maximum biodegradation efficiency 10.62% for C0 = 1.0 mg/mL | |||
| Ciprofloxacin | Maximum biodegradation efficiency 2.45% for C0 = 0.6 mg/mL | |||
| Bacterial community composed of Desulfovibrio, Enterococcus and Peptostreeptococcus spp. (active cells) | Ciprofloxacin | sulfate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 85%, after 6 days | [64] |
| Bacterial community composed of Comamonas, Arcobacter, Dysgonomonas, Macellibacteroides and Actinomyces, genera (active cells) | Ciprofloxacin | nitrate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 83%, after 6 days | [64] |
| Acinetobacter sp. (active cells) | Sulfamethoxazole | pH = 7.0; temperature = 25 °C in the dark; time = 6 days; initial concentration of pharmaceutical compound = 30.0 mg/L; in a shaker at 150 rpm; batch system | Biodegradation efficiency was 98.8%, after 10 h | [51] |
| Bradyrhizobium sp. GLC_01 strain (active cells) | Ciprofloxacin | biodegradation via cometabolism with another carbon substrate (glucose and sodium acetate); temperature = 25 °C; time = 8 days; initial concentration of pharmaceutical compound = 0.05 ÷ 10 mg/L; in a rotatory shaker at 150 rpm; batch system | Over 70% biodegradation was achieved at 0.05 mg/L whereas decreased to 26% at 10 mg/L | [69] |
| Bacillus subtilis strain (active cells) | Cephalexin | pH = 6.5; temperature = 35 °C; time = 12 days; initial concentration of pharmaceutical compound = 1.0 g/L; batch system | Biodegradation potential was 27, 22 and 21% in the presence of Ni2+, Cu2+, Zn2+ ions in solution at 10 mg/L concentration | [50] |
| Bacterial consortium composed of Acinetobacter lwoffii ACRH76, Bacillus pumulis C2A1, and Acinetobacter sp. HN3) (active cells immobilized or in suspension) | Ciprofloxacin | environmental conditions; temperature = 30 ± 2 °C; time = 20 days; initial concentration of pharmaceutical compound = 100.00 mg/L; floating treatment wetland strategy (FTWs) | Maximum biodegradation was 97% in the FTWs having immobilized bacteria | [70] |
| Achromobacter sp. JL9 with in-situ generated biogenic manganese oxides (active cells) | Sulfamethoxazole | pH = 7.0; temperature = 30 ± 1 °C; time = 84 h; initial concentration of pharmaceutical compound = 5.0 mg/L; in a shaker at 125 rpm; batch system | Maximum biodegradation was 97.43% for the Mn (II) concentration of 2 mg/L | [71] |
| Microbial community including Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Armatimonadetes (active cells) | Chlortetracycline | pH = 7.2; temperature = 5 ÷ 45 °C; time = 28 days; initial con-centration of pharmaceutical compound = 100 µg/L; aerobic conditions; in a shaker at 120 rpm; batch system | Biodegradation rates of 48.7% and 84.9% were achieved by acclimated microbial populations in one and four weeks, respectively for the initial chlortetracycline level of 100 µg/L | [72] |
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Sulfamethoxazole | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodegradation of 73.2 ± 21.3% were achieved by simultaneous removal of drugs | [73] |
| Pseudomonas sp. CE22 strain isolated from activated sludge (active cells) | Cephalexin | temperature =26 °C; time = 10 h; initial concentration of pharmaceutical compound = 10 mg/L; in a shaker at 200 rpm; batch system | Biodegradation over 90% after incubation for 10 h | [74] |
| Activated sludge bacteria (inactivated biomass) | Ofloxacin | pH = 7.0; temperature =25 °C; time = 48 h; initial concentration of pharmaceutical compounds = 100 ÷ 700 ng/mL; in an orbital shaker at 120 rpm; batch system | Removal efficiency 45% for C0 = 100 ng/mL and 21% for C0 = 700 ng/mL; maximum biosorbtion capacity 1.5 ± 0.03 mg/g TSS | [75] |
| Norfloxacin | Removal efficiency 50% for C0 = 100 ng/mL and 39% for C0 = 700 ng/mL; maximum biosorbtion capacity 3.24 ± 0.05 mg/g TSS | |||
| Ciprofloxacin | Removal efficiency 59% for C0 = 100 ng/mL and 43% for C0 = 700 ng/mL; maximum biosorbtion capacity 3.39 ± 0.06 mg/g TSS | |||
| Bacterial consortium of Burkholderia cepacia, Chrysomonas luteola, Pseudomonas fluorescens, Bacillus subtilis, Bacillus megaterium, Bacillus sterothermophilus, Citrobacter freundii, Kluyvera (active and inactive cells) | Cephalexin | pH = 6.0; temperature =25 °C; time = 90 min; initial concentration of pharmaceutical compound = 0.2 ÷ 5.0 mg/L; in an orbital shaker at 125 rpm; batch system | Maximum biosorption efficiency (94.73% vs. 92.98% for living and dead cells respectively) was recorded at C0 = 0.4 mg/L, while for C0 = 5 mg/L dead cells exhibited more efficiency compared with living cells (82.36% vs. 46.66% respectively) | [46] |
| Antipyretics and analgesics | ||||
| Pseudomonas PrS10 strain (active cells) | Paracetamol | pH = 7.2 ÷ 7.4; temperature =30 °C; time = 4–7 days; initial concentration of pharmaceutical compound = 3 g/L; in an orbital shaker at 140 rpm; batch system | Maximum biodegradation efficiency 96.37%, with 4.8 g/L of carbohydrate added, after 7 days | [16] |
| Anti-inflammatories | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Ibuprofen | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodeg-radation of 24.2 ± 14.6% were achieved by simultaneous removal of drugs | [73] |
| Nitrifying bacteria isolated from activated sludge (active cells) | Ibuprofen | pH at approximately 7.5–8.0 during the incubation period; temperature =25 °C; initial concentration of pharmaceutical compounds = 25 ÷ 200 µg/L; in an orbital shaker at 80 rpm; batch system | Complete biodegradation (100%) at the lower concentration levels (25–100 µg/L) in 24 h | [76] |
| Ketoprofen | Complete biodegradation (100%) at the lower concentration levels (25–100 µg/L) in 150 h | |||
| Activated sludge bacteria (active cells) | Ibuprofen | pH = 7.0; initial concentration of pharmaceutical compound = 50 ÷ 300 mg/L; temperature =30 °C; in a shaker at 100 rpm in the dark; aerobic conditions; batch system | Biodegradation to undetectable concentrations within 4 days for C0 = 250 mg/L | [77] |
| Diclofenac | Biodegradation rate of 75% within 3 weeks for C0 = 300 mg/L | |||
| Brevibacterium sp. D4 strain (active cells) | Diclofenac | temperature =25 °C; initial concentration of pharmaceutical compounds = 10 mg/L; time = 30 days; in an orbital shaker at 150 rpm; batch system | Biodegradation was 35% for C0 = 10 mg/L of drug as a sole carbon source; Periodic feeding with acetate as a supplementary carbon source increased biodegradation by up to 90% | [78] |
| Anti-epileptics | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Carbamazepine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by bio-degradation of 4.2 ± 2.3% were achieved by simultaneous removal of drugs | [73] |
| Starkeya sp. C11 strain and Rhizobium sp. C12 strain (active cells) | Carbamazepine | temperature =25 °C; initial concentration of pharmaceutical compounds = 10 mg/L; time = 30 days; in an orbital shaker at 150 rpm; batch system | Biodegradation was 30% for C0 = 10 mg/L of drug as a sole carbon source | [78] |
| Antidepressants | ||||
| Labrys portucalensis F11 strain (active cells) | Fluoxetine (racemic mixture) and its enantiomers FLX | temperature = 25 °C; initial concentration of pharmaceutical compounds = 2 µM ÷ 21 µM; time = 56 days; in an orbital shaker at 130 rpm; protected from light; batch system | Complete biodegradation of both enantiomers at C0 = 2 µM for FLX as sole carbon source was achieved in 30 days; The enantiomers were partially degraded at initial concentrations of 4 and 9 µM. Complete biodegradation of the two enantiomers occurred in the presence of acetate as an additional carbon source at 4, 9, and 21 µM | [79] |
| Antiseptics | ||||
| Enterobacter hormaechei ssp. Xiangfangensis KG216S strain (active cells) | Basic fuchsine | temperature = 37 ± 2 °C; initial concentration of pharmaceutical compounds = 20 ÷ 100 mg/L; time = 72 h; in an orbital shaker at 100 rpm; batch system | Maximum biosorption capacity was 140.54 mg/g for C0 = 20 mg/L, with 4 g/L of sucrose added | [80] |
| Histamine-2 blockers | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Ranitidine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic conditions | Removal rates by biodegradation and biosorption of 60.8 ± 15.0% were achieved by simultaneous removal of drugs | [73] |
| Hormones | ||||
| Bacterial community composed of Comamonas, Arcobacter, Dysgonomonas, Macellibacteroides and Actinomyces, genera (active cells) | 17β-estradiol | nitrate-reducing conditions; temperature = 25 ± 2 °C in the dark; time = 6 days; initial concentration of pharmaceutical compounds = 1.0 mg/L; anaerobic conditions; batch system | Biodegradation efficiency was 84%, after 6 days | [64] |
| Psycho-stimulants | ||||
| Mixed culture of heterotrophic bacteria from activated sludge from sewage treatment plants (active cells) | Caffeine | pH = 7.0; initial concentration of pharmaceutical compound = 20 ÷ 50 µg/L; suspended growth reactor SGR with stirring at 400 rpm; 24-cycle biodegradation experiment in a single SGR; simultaneous removal of 5 drugs; aerobic condition | Removal rates by biodegradation and biosorption of 5.3 ± 4.4% were achieved by simultaneous removal of drugs | [73] |
| FUNGAL BIOMASS | ||||
| Antibiotics | ||||
| Saccharomyces cerevisiae (active cells) | Amoxicillin | pH = 2 ÷ 8, initial concentration of pharmaceutical compound = 5 ÷ 5 mg/L, the amount of biosorbent = 0.1 ÷ 1.5 g/L; contact time = 10 ÷ 240 min | The highest removal efficiency, 93%, was obtained for C0 = 5 mg/L, bioadsorbent dose 0.75 g/L, pH = 5, time = 120 min; the highest value of adsorption capacity was 12 mg/g in the same conditions | [45] |
| Trametes versicolor ATCC 42530 strain (active cells) | Sulfapyridine | pH = 4.5 ± 0.3; temperature = 25 °C; biomass dose 1.8 g/L (measured as dry weight); tank bioreactor with mechanical agitation at 115 rpm; 5 g/L of glucose added; aeration condition; batch model | Complete biodegradation (100%) for C0 = 21.4 ng/g within 26 days | [66] |
| Sulfathiazole | Removal efficiency by biodegradation was 85.9% for C0 = 143.0 ng/g within 26 days | |||
| Anti-inflammatories | ||||
| Trametes versicolor ATCC 7731 strain (living cells and chemically inactivated cells) | Naproxen | pH = 4.0; temperature = 25 °C; initial concentration of pharmaceutical compounds = 50–100 μg/L; biomass dose 0.4 g/L (measured as freshly grown fungus culture); time = 24 h; rotary shaker at 70 rpm; batch model | Biodegradation efficiency was over 60% with living cells and biosorption efficiency was 14 ± 5% with inactivated cells | [81] |
| Ibuprofen | Biodegradation efficiency was over 60% with living cells and biosorption efficiency was 32 ± 1% with inactivated cells | |||
| Trametes versicolor Ganoderma lucidum (active cells) | Diclofenac | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | The overall removal (100%) of diclofenac and ibuprofen after 7 days of incubation were achieved by both strains, T. versicolor and G. lucidum, by abiotic, biosorption and biodegradation | [82] |
| Ibuprofen | ||||
| Trametes versicolor Irpex lacteus Trichoderma reesei (active cells) | Diclofenac | pH = 5.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 2.5 ÷ 5 mg/L; time = 3 h ÷ 14 days; shaking incubator (150 rpm); batch model and fungal biofilm | T. versicolor and I. lacteus was able to completely (>99.9%) remove diclofenac after 7 days, by both mechanisms: enzyme activity and biosorption | [83] |
| Fusarium solani Pleurotus ostreatus (active cells) | Diclofenac | F. solani indicated a maximum reduction of 90% of diclofenac after 21 days; P. ostreatus removed the diclofenac >99.9% after 14 days; The combination of F. solani and P. ostreatus showed >80% removal of diclofenac after 14 days | [83] | |
| Trametes versicolor (active cells) | Ketoprofen | Only T. versicolor was able to reduce more than 80% of ketoprofen after 21 days of incubation | [83] | |
| Phanerochaete chrysosporium (active cells) | Naproxen | pH = 3.2 ÷ 4.5; temperature = 30 °C; initial concentration of pharmaceutical compound = 1.0 mg/L; continuous aerating mode; time = 28 days; batch system | Removal efficiency was 80.55 ± 3.26 on day 7; A removal higher than 95% was achieved after the addition of 8.25% sodium hypochlorite for inhibiting contamination in the reactor, on day 21; More than 90% naproxen C0 = 10 mg/L) was removed by the crude enzyme in the first two days | [84] |
| Ganoderma lucidum (FP-58537-Sp strain) (active cells) | Diclofenac | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 98 ± 15% of which 58 ± 8% by biodegradation and 40 ± 6%by biosorption | [63] |
| Anti-epileptics | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Carbamazepine | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Maximum removal was 32%, achieved by biosorption, using the combined fungal system | [82] |
| Trametes versicolor (active cells) | Carbamazepine | pH = 7.5; temperature = 34–37 °C; initial concentration of pharmaceutical compound = 1 ÷ 20 mg/L; time = 5 h–7 d; first batch operation, then a continuous mode | Around 80% was eliminated when the diluted synthetic medium was applied as feeding. An effective elimination was achieved in ~100 days continuous operation, if sufficient nutrients were supplied | [85] |
| Phanerochaete chrysosporium (active cells) | Carbamazepine | pH = 3.2 ÷ 4.5; temperature = 30 °C; initial concentration of pharmaceutical compound = 1.0 mg/L; time = 28 days; aerating mode; batch system | Removal efficiency was 32.55 ± 1.22% on day 7 | [84] |
| Stropharia rugosoannulata (FBCC 475 strain) (active cells) | Carbamazepine | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 86 ± 7%, of which 84 ± 7% by biodegradation and 2% by biosorption | [63] |
| Ganoderma lucidum (FP-58537-Sp strain) (active cells) | Carbamazepine | Total removal was 36 ± 7%, of which 31 ± 6% by biodegradation and 5 ± 1% by biosorption | [63] | |
| Antidepressants | ||||
| Trametes versicolor (ATCC #42,530 strain) (active cells) | Venlafaxine | pH = 4.5 ± 0.5; temperature = 25 °C; initial concentration of pharmaceutical compound = 47 ÷ 184 μg/L; time = 6–26 days; first batch system, orbital shaking (135 rpm), dark conditions | Total removal was 55 ± 8%, of which 53 ± 8% by biodegradation and 2% by biosorption | [63] |
| Lipid regulators | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Gemfibrozil | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Complete removal (100%) is attributed to high intracellular oxidative biological pathway | [82] |
| Clofibric acid | Removal efficiency was 14%, achieved by biosorption with T. versicolor strain and 41% with both strains simultaneously | |||
| Hormones | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Progesterone | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Total removal (100%) of progesterone was achieved by both strains, T. versicolor and G. lucidum, predominantly through biodegradation | [82] |
| Histamine-2 blockers | ||||
| Trametes versicolor Ganoderma lucidum (active cells) | Ranitidine | pH = 4.5; temperature = 25 °C; initial concentration of each pharmaceutical compounds = 50 μg/L; biomass dose 1 g/L (measured as dry weight); time = 7 days; shaken conditions (150 rpm); batch model | Total removal (100%) of ranitidine was mainly attributed to biological removal of the live fungal biomass by intra- or extracellular oxidative mechanisms | [82] |
4.2. Biosorbents Based on Natural Polymers
| Biosorbent | Therapeutic Group/ Pharmaceutical Compound | Process Parameters | Obtained Results | Ref. |
|---|---|---|---|---|
| ALGINATE | ||||
| Antibiotics | ||||
| Alginate-graphene-ZIF67 aerogel (AG-ZIF) Alginate-graphene-Co aerogel (AG-Co) | Tetracycline | pH = 6.0; room temperature; time = 720 min; initial concentration of pharmaceutical compound = 100 mg/L; adsorbent dose = 1 g/L; shaker at 150 rpm; batch system | Maximum adsorption capacities for AG-ZIF and AG-Co were 456.62 and 105.49 mg/g, respectively | [92] |
| Ga-based metal-organic gel/sodium alginate composite beads | Chlortetracycline hydrochloride | pH = 4.0 ÷ 8.0; temperature = 25 °C; initial concentration of each pharmaceutical compound = 20 mg/L; adsorbent dose = 1.00 g/L (measured as dry weight); time = 72 h; shaken conditions (150 rpm); batch model; dark environment | Maximum adsorption capacity was 1085.19 mg/g | [93] |
| Ciprofloxacin hydrochloride | Maximum adsorption capacity was 862.07 mg/g | [93] | ||
| Polyvinyl alcohol-copper alginate gel beads | Tetracycline | pH = 3 ÷ 11; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20 mg/L; adsorbent dose = 0.2 ÷ 2 g/L, time = 24 h; shaker at 150 rpm; batch system | Removal efficiency was 97.8% and the maximum adsorption capacity was 231.431 mg/g, when the dose of adsorbent is 2 g/L and temperature = 45 °C | [94] |
| Anti-inflammatories | ||||
| Alginate/ Carbon-based films | Diclofenac | pH = 3 ÷ 11; temperature = 30–68 °C; initial concentration of each pharmaceutical compound = 10 ÷ 50 mg/L; adsorbent dose = 0.25÷2.0 g/L, time = 6 h; batch system under stirring of 400 rpm | Maximum DCF adsorption of 29.9 mg/g was obtained at pH = 3 and 30 °C | [95] |
| Alginate/polypyrrole/ ZnFe2O4 beads | Ibuprofen | pH = 5 ÷ 7; initial concentration of the pharmaceutical compound = 50–350 mg/L; beads dosage = 0.2–1 g/L (dry weight); temperature = 25–55 °C; contact time = 3 h; orbital shaking 150 rpm; batch system | Maximum adsorption capacity was 108.2 mg/g, that increase with 12% under an external magnetic field (EMF); C0 = 350 mg/L; adsorbent dose = 0.2 g/L, pH = 7.0, temperature = 25 °C; stirring 100 rpm | [96] |
| Antipyretics and analgesics | ||||
| Calcium alginate/activated hydrochar composite beads | Paracetamol | pH = 6.5; initial concentration of the pharmaceutical compound = 100–250 mg/L; temperature = 15–35 °C; time = 10 h; shaker 150 rpm; batch system | Maximum adsorption capacity was 165.94 mg/g, for C0 = 250 mg/L, after 4 h at temperature = 25 °C | [97] |
| Alginate/polypyrrole/ ZnFe2O4 beads | Paracetamol | pH = 5 ÷ 7; initial concentration of the pharmaceutical compound = 50–350 mg/L; beads dosage = 0.2–1 g/L (dry weight); temperature = 25–55 °C; contact time = 3 h; orbital shaking 150 rpm; batch system | Maximum adsorption capacity was 106.7 mg/g, that increase with 14% under an EMF; C0 = 350 mg/L; adsorbent dose 0.2 g/L, pH = 7.0, temperature = 25 °C; stirring 100 rpm | [96] |
| Biomarkers | ||||
| Alginate-graphene composites | Rhodamine B | initial concentration of the pharmaceutical compound = 5 mg/L; contact time = 24 h; darkness and gentle stirring; batch system | Maximum adsorption capacity of 178 mg/g was achieved for reduced graphene oxide-based beads | [98] |
| Antiretroviral | ||||
| Activated carbon encapsulated in sodium alginate | Tenofovir disoproxil fumarate | pH = 4, an initial TDF concentration = 0.1 mM; temperature = 25 °C; adsorbent dose = 1 g of wet beads to 10 mL of TDF solution, the beads were whirled at 350 rpm | Maximum removal efficiency by adsorption was 92.68% after a contact time of 120 min | [99] |
| CHITOSAN | ||||
| Antibiotics | ||||
| Chitosan-alginate-bentonite composites | Tetracycline | pH = 5.5; temperature = 25–50 °C; initial concentration of each pharmaceutical compound = 10–550 mg/L; adsorbent dose = 1–4 g/L; time = 240 min; batch system under continuous agitation | Adsorption efficiency was 97.7% for C0 = 10 mg/L at 50 °C after 30 min | [100] |
| Chitosan | Rifampicin | pH = 6.7; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5–10 g/L; time = 240 min; orbital shaker (100 rpm); batch technique | Maximum adsorption capacity was 66.91 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] |
| Streptomycin | Maximum adsorption capacity was 11.00 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] | ||
| Chitosan-based magnetic composite | Tetracycline hydrochloride | pH = 4 ÷ 12; temperature = 15–35 °C; initial concentration of pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5 g (dry weight); time = 12 h; shaking speed of 140 rpm; dark environment; batch system | Maximum adsorption capacity was 50.2 mg/g for C0 = 100 mg/L at pH = 10 and temperature = 25 °C | [102] |
| Metal and clay embedded cross-linked chitosan | Tetracycline | pH = 2 ÷ 12; temperature = 25–45 °C; initial concentration of pharmaceutical compound = 20 mg/L; adsorbent dose = 0.5 g (dry weight); time = 24 h; orbital shaker 140 rpm; dark environment; batch system | Maximum adsorption capacity was 104.17 mg/g using zirconium loaded chitosan modified by perlite (Zr/Cht/Pt) composite at pH = 4; temperature = 25 °C | [103] |
| CuCoFe2O4—Chitosan magnetic nanohybrid | Tetracycline | pH = 3.5 ÷ 11.5; temperature = 25–40 °C; initial concentration of pharmaceutical compound = 5–30 mg/L; adsorbent dose = 0.2–1 g/L; time = 30 min; batch system | Highest adsorption efficiency was 93.07% for C0 = 5 mg/L, pH = 3.5, contact time of 20 min, the dose of 0.4 g/ L, and temperature of 25 °C | [104] |
| Chitosan-curdlan composite magnetized by zinc ferrite | Tetracycline | pH = 1.0 ÷ 11.0; temperature = 10–65 °C; initial concentration of pharmaceutical compound = 20–160 mg/L; adsorbent dose = 0.25–0.85 g/L; time = 120 min; batch system | Maximum adsorption capacity was 371.42 mg/g at 55 °C, C0 = 160 mg/L, 0.65 g/L dosage of adsorbent and pH = 6 | [105] |
| Chitosan-carbon black waste composite beads | Amoxicillin | pH = 6.5 ÷ 8.5; temperature = 22 °C; initial concentration of pharmaceutical compound = 25–50 mg/L; composite beads dose = 10–20 g/L; time = 24 h; batch system | Maximum adsorption capacity was 12 mg/g for C0 = 25 mg/L (in demineralized water) and 15 mg/g for C0 = 25 mg/L (in tap water); pH = 6.5 ÷ 7.5 | [106] |
| Chitosan-carbon black waste composite beads | Tetracycline | Maximum adsorption capacity was 39 mg/g for C0 = 25 mg/L (in demineralized water) at pH = 7.5 ÷ 8.5 | [106] | |
| Anti-inflammatories | ||||
| Chitosan | Ibuprofen | pH = 6.7; temperature = 20–45 °C; initial concentration of each pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5–10 g/L; time = 240 min; orbital shaker (100 rpm); batch system | Maximum adsorption capacity was 24.21 mg/g for C0 = 30 mg/L and adsorbent dose = 1.5 g/L | [101] |
| Chitosan-based magnetic composite | Diclofenac sodium | pH = 4 ÷ 12; temperature = 15–35 °C; initial concentration of pharmaceutical compound = 20–200 mg/L; adsorbent dose = 0.5 g (dry weight); time = 12 h; shaking speed of 140 rpm; dark environment; batch system | Maximum adsorption capacity was 123 mg/g for C0 = 100 mg/L at pH = 6 and temperature = 25 °C | [102] |
| Chitosan/Zr-MOF (UiO-66) composite foams | Ketoprofen | pH = 2 ÷ 9; initial concentration of pharmaceutical compound = 5–50 mg/L; adsorbent dose = 0.2 g/L; time = 10 h; shaking speed of 180 rpm; batch system | Maximum adsorption capacity of 209.7 mg/g was achieved for C0 = 50 mg/L at pH 4 | [107] |
| Magnetic Fe/Cu–alginate nanocomposite beads | Naproxen | pH = 5.0; temperature = 25 °C; initial concentration of each pharmaceutical compound = 25 mg/L; adsorbent dose = 25 mg/30 mL of drug solutions; mixed at 250 rpm for 10 min; batch system | Removal efficiency was 84% for C0 = 25 mg/L and adsorbent dose = 25 mg/50 mL | [108] |
| Magnetic Fe/Cu-chitosan nanocomposite beads | Diclofenac sodium | Removal efficiency was 92% for C0 = 25 mg/L and adsorbent dose = 25 mg/50 mL | [108] | |
| Psycho-stimulants | ||||
| Chitosan/activated carbon composite beads | Caffeine | natural pH; temperature = 25 °C; initial concentration of each pharmaceutical compound = 50–800 mg/L; adsorbent dose = 1 g/L; time = 48 h; orbital shaker at 150 rpm; batch system | Maximum adsorption capacity was 391.00 mg/g for C0 = 10 mg/L | [109] |
| Non-ergot dopamine agonists | ||||
| Chitosan grafted with sulfonic acid | Pramipexole | pH = 10; temperature = 25 °C; initial concentration of the pharmaceutical compound = 0–500 mg/L; adsorbent dose = 1 g/L; orbital shaker at 160 rpm; time = 24 h; batch system | Maximum adsorption capacity was 339 mg/g for C0 = 10 mg/L in the presence of 20 mg/L humic acid | [110] |
| Hormones | ||||
| Chitosan nanoparticles | Estrogen | pH = 7.3; estrogen initial concentration = 3.5 ÷ 11.5 mg/L; adsorbent dosage = 1.45 g/L; time = 300 min. and magnetic stirrer at 600 rpm; batch system | Removal efficiency was 92.50% for C0 = 5.7 mg/L and contact time of 220 min | [111] |
| OTHER NATURAL POLYMERS | ||||
| Anti-epileptics | ||||
| Ball-Milled Silk Fibroin Films | Carbamazepine | pH = 2–12; temperature = 15–45 °C; initial concentration of the pharmaceutical compound = 250 μg/L; time = 180 min; agitation speed of 100 rpm; batch system | Removal efficiency was 53% and adsorption capacity = 281 μg/g at pH = 12 | [112] |
| Antibiotics | ||||
| Heterogeneous natural polymer-based on dialdehyde inulin and laccase | Ofloxacin | pH = 4.5; temperature = 40 °C; initial concentration of the pharmaceutical compound = 25 mM; adsorbent dose = 8.5 mg/mL of the immobilized laccase; time = 60 h; stirring; batch system | Removal efficiency by biodegradation was 63% after 60 h of incubation | [113] |
| Biomarkers | ||||
| Gelatin/activated carbon composite | Rhodamine B | pH = 2–11; temperature = 30–60 °C; initial concentration of the pharmaceutical compound = 50–50 mg/L; adsorbent dose = 3 g/L; time = 42 h; agitation speed of 100 rpm; batch system | Maximum adsorption capacity was 256.41 mg/g for C0 = 5.7 mg/L; pH = 4; temperature = 30 °C; time = 27 h | [114,115] |
| Poly(lactic acid)/activated carbon | Rhodamine B | pH = 2–12; temperature = 30–60 °C; initial concentration of the pharmaceutical compound = 100 mg/L; adsorbent dose = 2 ÷ 10 g/L; time = 54 h; agitation speed of 100 rpm; batch system | Removal efficiency was 88.99%; a highest adsorption capacity was 149.57 mg/g at pH = 4 and temperature = 60 °C | [116] |
4.3. Biosorbents Based on Microbial Biomass Immobilized in Natural Polymers
- – In entrapment, microorganisms are physically entrapped in a porous matrix or gel suitable for different types of cells. They are usually mixed with a gel-forming material such as calcium alginate, agar, or polyacrylamide, which solidifies into beads or capsules and immobilizes the microorganisms, allowing good mass transfer.
- – In adsorption, microorganisms are bound to a solid support material by non-covalent interactions such as electrostatic forces, hydrogen bonds, or hydrophobic interactions. The support material can be in the form of particles, fibers, or a thin layer. Immobilization by adsorption is relatively easy, but the microorganisms may be desorbed over time.
- – In covalent bonding, the microorganisms are chemically bound to the support material using cross-linking agents or functional groups. This method offers stronger and more stable bonding compared with adsorption but requires specific reactive groups on the microorganisms and the support material for successful bonding.
- – In encapsulation, the microorganisms are enclosed in a semi-permeable membrane, or microcapsule. The membrane allows the diffusion of contaminants while protecting the microorganisms from external factors. Common encapsulation materials include polymers such as alginate, chitosan, or various gums. Encapsulation provides good protection for the microorganisms but can restrict mass transfer.
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Ahmad, A.; Priyadarshini, M.; Das, S.; Ghangrekar, M.M. Electrocoagulation as an efficacious technology for the treatment of wastewater containing active pharmaceutical compounds: A review. Sep. Sci. Technol. 2021, 57, 1234–1256. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment—Occurrence and environmental implications. Eur. J. Pharmacol. 2019, 866, 172813. [Google Scholar] [CrossRef]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Uribe, I.O.; Mosquera-Corral, A.; Rodicio, J.L.; Esplugas, S. Advanced Technologies for Water Treatment and Reuse; Wiley Online Library: Hoboken, NJ, USA, 2015; Volume 61, pp. 3146–3158. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; (Brazdis), R.I.M.; Manaila-Maximean, D. Natural and Natural-Based Polymers: Recent Developments in Management of Emerging Pollutants. Polymers 2023, 15, 2063. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Morosanu, I.; Teodosiu, C.; Fighir, D.; Paduraru, C. Simultaneous biosorption of micropollutants from aqueous effluents by rapeseed waste. Process. Saf. Environ. Prot. 2019, 132, 231–239. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L. Removal of methylene blue dye from aqueous solution using seashell wastes as biosorbent. Environ. Eng. Manag. J. 2012, 11, 1977–1985. [Google Scholar] [CrossRef]
- Rusu, L.; Harja, M.; Munteanu, C.; Ciobanu, G.; Suteu, D. Red and brown peat use in removing pollutants from municipal and industrial wastewater. J. Environ. Prot. Ecol. 2014, 15, 1690–1699. [Google Scholar]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers 2022, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol biodegradation by Pseudomonas strain PrS10 isolated from pharmaceutical effluents. Int. Biodeterior. Biodegrad. 2022, 175, 105490. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A. Sub-lethal effects induced by a mixture of three non-steroidal anti-inflammatory drugs (NSAIDs) on the freshwater bivalve Dreissena polymorpha. Ecotoxicology 2011, 21, 379–392. [Google Scholar] [CrossRef]
- Ramos, A.; Correia, A.; Antunes, S.; Gonçalves, F.; Nunes, B. Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: Detoxification mechanisms, oxidative defence system and peroxidative damage. Environ. Toxicol. Pharmacol. 2014, 37, 1221–1228. [Google Scholar] [CrossRef]
- Basol, N.; Ozmen, C.; Ocakli, S.; Cetin, S. Evaluation of the effects of curcumin, erdosteine, vitamin E and vitamin C on paracetamol toxicity. Medicine 2022, 11, 465. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A.; Oderinde, R.A. Chemically modified vermiculite clay: A means to remove emerging contaminant from polluted water system in developing nation. Polym. Bull. 2019, 76, 4967–4989. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, C.; Zheng, X.; Liu, S.; Hu, F.; Xu, L.; Xu, G.; Peng, X. Removal of Ciprofloxacin with Aluminum-Pillared Kaolin Sodium Alginate Beads (CA-Al-KABs): Kinetics, Isotherms, and BBD Model. Water 2020, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Vrinceanu, N.; Hlihor, R.M.; Simion, A.I.; Rusu, L.; Fekete-Kertész, I.; Barka, N.; Favier, L. New Evidence of the Enhanced Elimination of a Persistent Drug Used as a Lipid Absorption Inhibitor by Advanced Oxidation with UV-A and Nanosized Catalysts. Catalysts 2019, 9, 761. [Google Scholar] [CrossRef] [Green Version]
- Favier, L.; Rusu, L.; Simion, A.I.; Hlihor, R.M.; Pacala, M.L.; Augustyniak, A. Efficient degradation of clofibric acid by heterogeneous photocatalytic oxidation process. Environ. Eng. Manag. J. 2019, 18, 1683–1692. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Vallejo, M.; Román, M.F.S.; Ortiz, I. Insight on the fundamentals of advanced oxidation processes. Role and review of the determination methods of reactive oxygen species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Napierska, D.; Sanseverino, I.; Loos, R.; Cortés, L.G.; Niegowska, M.; Lettieri, T. Modes of action of the current Priority Substances list under the Water Framework Directive and other substances of interest. Publ. Off. Eur. Union. Doi 2018, 10, 226911. [Google Scholar]
- Pal, R.; Megharaj, M.; Kirkbride, K.P.; Naidu, R. Illicit drugs and the environment—A review. Sci. Total Environ. 2013, 463, 1079–1092. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Your Conclusions Valid? A Critical Review. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S. Review: Pharmacological Pollution in Water. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1074–1116. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation Characteristics of Pharmaceutical Substances by Whole Fungal Culture Trametes versicolor and its Laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.; Silva, A.M. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Noman, E.A.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Talip, B.A.; Hossain, S.; Altowayti, W.A.H.; Ismail, N. Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: A critical review. J. Hazard. Mater. 2021, 417, 126040. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; El-Monaem, E.M.A.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2021, 15, 103543. [Google Scholar] [CrossRef]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: Cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis EA, S.; Porras, J.; Flórez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef] [PubMed]
- Samarghandi, M.R.; Asgari, G.; Shokoohi, R.; Dargahi, A.; Arabkouhsar, A. Removing amoxicillin antibiotic from aqueous solutions by Saccharomyces cerevisiae yeast bioadsorbent: Kinetic, thermodynamic and isotherm studies. Desalination Water Treat. 2019, 152, 306–315. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Efaq, A.; Mohamed, R.; Norli, I.; Kadir, M. Potential of bacterial consortium for removal of cephalexin from aqueous solution. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kang, Y.; Yin, W.; Fan, J.; Guo, Z. Removal of Antibiotics from Aqueous Solutions by a Carbon Adsorbent Derived from Protein-Waste-Doped Biomass. ACS Omega 2020, 5, 19187–19193. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.M.; Șuteu, D.; Harja, M. Application of Saccharomyces cerevisiae/Calcium Alginate Composite Beads for Cephalexin Antibiotic Biosorption from Aqueous Solutions. Materials 2021, 14, 4728. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Istrate, B.; Harja, M. Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers 2022, 14, 2855. [Google Scholar] [CrossRef]
- Adel, A.; Lalung, J.; Efaq, A.; Ismail, N. Removal of cephalexin antibiotic and heavy metals from pharmaceutical effluents using Bacillus subtilis strain. Expert Opin. Environ. Biol. 2015, 4, 2. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Wang, J. Biodegradation of typical pharmaceutical compounds by a novel strain Acinetobacter sp. J. Environ. Manag. 2018, 217, 240–246. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Botezatu, A.V.D.; Istrate, B.; Doroftei, I. Eco-Friendly Biosorbents Based on Microbial Biomass and Natural Polymers: Synthesis, Characterization and Application for the Removal of Drugs and Dyes from Aqueous Solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Schnakovszky, C.; Favier, L. Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies. Polymers 2022, 14, 3388. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption—An alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.; Saravanan, A.; Kumar, P.S.; Yaashikaa, P.; Thamarai, P.; Shaji, A.; Rangasamy, G. A review on algae biosorption for the removal of hazardous pollutants from wastewater: Limiting factors, prospects and recommendations. Environ. Pollut. 2023, 327, 121572. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Rusu, L.; Harja, M.; Simion, A.I.; Suteu, D.; Ciobanu, G.; Favier, L. Removal of Astrazone Blue from aqueous solutions onto brown peat. Equilibrium and kinetics studies. Korean J. Chem. Eng. 2014, 31, 1008–1015. [Google Scholar] [CrossRef]
- Suteu, D.; Biliuta, G.; Rusu, L.; Coseri, S.; Nacu, G. Cellulose cellets as new type of adsorbent for the removal of dyes from aqueous media. Environ. Eng. Manag. J. 2015, 14, 525–532. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Wang, T. Cadmium biosorption by lactic acid bacteria Weissella viridescens ZY-6. Food Control 2020, 123, 107747. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef]
- Benjedim, S.; Romero-Cano, L.A.; Pérez-Cadenas, A.F.; Bautista-Toledo, M.I.; Lotfi, E.M.; Carrasco-Marín, F. Removal of emerging pollutants present in water using an E-coli biofilm supported onto activated carbons prepared from argan wastes: Adsorption studies in batch and fixed bed. Sci. Total Environ. 2020, 720, 137491. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yang, M.; Zhang, J.; Yang, B.; Lin, K.; Li, J.; Gan, J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard. Mater. 2017, 330, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Castellet-Rovira, F.; Villagrasa, M.; Badia-Fabregat, M.; Barceló, D.; Vicent, T.; Caminal, G.; Sarrà, M.; Rodríguez-Mozaz, S. The role of sorption processes in the removal of pharmaceuticals by fungal treatment of wastewater. Sci. Total. Environ. 2018, 610, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Sanches, S.; Pereira, I.A. Anaerobic biodegradation of pharmaceutical compounds: New insights into the pharmaceutical-degrading bacteria. J. Hazard. Mater. 2018, 357, 289–297. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.; Feijoo, G.; Moreira, M.; Lema, J. Operation of stirred tank reactors (STRs) and fixed-bed reactors (FBRs) with free and immobilized Phanerochaete chrysosporium for the continuous removal of pharmaceutical compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.E.; Barón, E.; Gago-Ferrero, P.; Jelić, A.; Llorca, M.; Farré, M.; Díaz-Cruz, M.S.; Eljarrat, E.; Petrović, M.; Caminal, G.; et al. Removal of pharmaceuticals, polybrominated flame retardants and UV-filters from sludge by the fungus Trametes versicolor in bioslurry reactor. J. Hazard. Mater. 2012, 233, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiang, Z.; Li, Y.; Ben, W. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics. J. Environ. Sci. 2017, 56, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.S.; Ismail, N. Biodegradation of Pharmaceutical Wastes in Treated Sewage Effluents by Bacillus subtilis 1556WTNC. Environ. Process. 2014, 1, 459–481. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Nghiem, L.D.; Oh, S. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge. Chemosphere 2018, 211, 600–607. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Rehman, M.U.; Tauseef, M.; Islam, E.; Hayat, A.; Iqbal, S.; Arslan, M.; Afzal, M. Ciprofloxacin Removal from Aqueous Media Using Floating Treatment Wetlands Supported by Immobilized Bacteria. Sustainability 2022, 14, 14216. [Google Scholar] [CrossRef]
- Liang, D.H.; Hu, Y.; Cheng, J.; Chen, Y. Enhanced performance of sulfamethoxazole degradation using Achromobacter sp. JL9 with in-situ generated biogenic manganese oxides. Bioresour. Technol. 2021, 333, 125089. [Google Scholar] [CrossRef]
- Liao, X.; Zou, R.; Li, B.; Tong, T.; Xie, S.; Yuan, B. Biodegradation of chlortetracycline by acclimated microbiota. Process. Saf. Environ. Prot. 2017, 109, 11–17. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Molina, R.; Martínez, F.; Melero, J.A. Biological removal of pharmaceutical and personal care products by a mixed microbial culture: Sorption, desorption and biodegradation. Biochem. Eng. J. 2013, 81, 108–119. [Google Scholar] [CrossRef]
- Lin, B.; Lyu, J.; Lyu, X.-J.; Yu, H.-Q.; Hu, Z.; Lam, J.C.; Lam, P.K. Characterization of cefalexin degradation capabilities of two Pseudomonas strains isolated from activated sludge. J. Hazard. Mater. 2015, 282, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.R.A.; Amorim, C.L.; Cravo, S.M.; Tiritan, M.E.; Castro, P.M.L.; Afonso, C.M.M. Fluoroquinolones biosorption onto microbial biomass: Activated sludge and aerobic granular sludge. Int. Biodeterior. Biodegrad. 2016, 110, 53–60. [Google Scholar] [CrossRef]
- Dawas-Massalha, A.; Gur-Reznik, S.; Lerman, S.; Sabbah, I.; Dosoretz, C.G. Co-metabolic oxidation of pharmaceutical compounds by a nitrifying bacterial enrichment. Bioresour. Technol. 2014, 167, 336–342. [Google Scholar] [CrossRef]
- Langenhoff, A.; Inderfurth, N.; Veuskens, T.; Schraa, G.; Blokland, M.; Kujawa-Roeleveld, K.; Rijnaarts, H. Microbial Removal of the Pharmaceutical Compounds Ibuprofen and Diclofenac from Wastewater. BioMed Res. Int. 2013, 2013, 325806. [Google Scholar] [CrossRef] [Green Version]
- Bessa, V.; Moreira, I.; Tiritan, M.; Castro, P. Enrichment of bacterial strains for the biodegradation of diclofenac and carbamazepine from activated sludge. Int. Biodeterior. Biodegrad. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Moreira, I.S.; Ribeiro, A.R.; Afonso, C.M.; Tiritan, M.E.; Castro, P.M. Enantioselective biodegradation of fluoxetine by the bacterial strain Labrys portucalensis F11. Chemosphere 2014, 111, 103–111. [Google Scholar] [CrossRef]
- Jathanna, N.N.; Krishnamurthy, G.K.; Paithankar, J.G.; Hegde, S.; Goveas, L.C.; Ravindranath, B.S.; Gowdru, M. Phyto-bacterial biosorption of basic fuchsine: A self-sustainable approach towards biomitigation of contaminant of emerging concern. J. Environ. Chem. Eng. 2023, 11, 109330. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters, industrial chemicals and pesticides by Trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Vasiliadou, I.; Sánchez-Vázquez, R.; Molina, R.; Martínez, F.; Melero, J.; Bautista, L.; Iglesias, J.; Morales, G. Biological removal of pharmaceutical compounds using white-rot fungi with concomitant FAME production of the residual biomass. J. Environ. Manag. 2016, 180, 228–237. [Google Scholar] [CrossRef]
- Dalecka, B.; Juhna, T.; Rajarao, G.K. Constructive use of filamentous fungi to remove pharmaceutical substances from wastewater. J. Water Process. Eng. 2020, 33, 100992. [Google Scholar] [CrossRef]
- Li, X.; Li, G. A review: Pharmaceutical wastewater treatment technology and research in China. In Proceedings of the Asia-Pacific Energy Equipment Engineering Research Conference, Zhuhai, China, 13–14 June 2015; Atlantis Press: Amsterdam, The Netherlands, 2015; pp. 345–348. [Google Scholar]
- Zhang, Y.; Geißen, S.-U. Elimination of carbamazepine in a non-sterile fungal bioreactor. Bioresour. Technol. 2012, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of Endocrine Disrupting Chemicals in Wastewater by Enzymatic Treatment with Fungal Laccases. Org. Process. Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Simelane, N.P.; Asante, J.K.; Ndibewu, P.P.; Mramba, A.S.; Sibali, L.L. Biopolymer composites for removal of toxic organic compounds in pharmaceutical effluents—A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100239. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Kofen, M. Synthesis and characterization of new insensitive and high-energy dense cellulosic biopolymers. Fuel 2021, 292, 120347. [Google Scholar] [CrossRef]
- Gowthaman, N.; Lim, H.; Sreeraj, T.; Amalraj, A.; Gopi, S. Advantages of biopolymers over synthetic polymers: Social, economic, and environmental aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Zia, Z.; Hartland, A.; Mucalo, M.R. Use of low-cost biopolymers and biopolymeric composite systems for heavy metal removal from water. Int. J. Environ. Sci. Technol. 2020, 17, 4389–4406. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, K.; Shi, B. Enhanced tetracycline adsorption using alginate-graphene-ZIF67 aerogel. Colloids Surf. A Physicochem. Eng. Asp. 2019, 588, 124360. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Z.; Jia, A.; Wang, Y.; Li, N.; Liu, Z.; Li, Y.; Zhang, H. New insight into adsorption and co-adsorption of chlortetracycline hydrochloride and ciprofloxacin hydrochloride by Ga-based metal-organic gel/sodium alginate composite beads. Sep. Purif. Technol. 2023, 312, 123408. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2021, 422, 126863. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Performance and interactions of diclofenac adsorption using Alginate/Carbon-based Films: Experimental investigation and statistical physics modelling. Chem. Eng. J. 2021, 428, 131929. [Google Scholar] [CrossRef]
- Silva, E.C.; Soares, V.R.; Fajardo, A.R. Removal of pharmaceuticals from aqueous medium by alginate/polypyrrole/ZnFe2O4 beads via magnetic field enhanced adsorption. Chemosphere 2023, 316, 137734. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; Dos Santos, D.F.; da Silva Fonseca, B.C.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen removal by calcium alginate/activated hydrochar composite beads: Batch and fixed-bed studies. International J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Tunioli, F.; Khaliha, S.; Mantovani, S.; Bianchi, A.; Kovtun, A.; Xia, Z.; Bafqi, M.S.S.; Okan, B.S.; Marforio, T.D.; Calvaresi, M.; et al. Adsorption of emerging contaminants by graphene related materials and their alginate composite hydrogels. J. Environ. Chem. Eng. 2023, 11, 109566. [Google Scholar] [CrossRef]
- Ajebli, S.; Kaichouh, G.; Khachani, M.; Babas, H.; EL Karbane, M.; Safi, Z.S.; Berisha, A.; Mehmeti, V.; Warad, I.; Zarrouk, A.; et al. Modeling of tenofovir disoproxil fumarate decontamination using sodium alginate-encapsulated activated carbon: Molecular dynamics, monte carlo and density functional theory. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131057. [Google Scholar] [CrossRef]
- Filho, F.G.N.; Filho, E.C.S.; Osajima, J.A.; de Melo Alves, A.P.; Fonseca, M.G. Adsorption of tetracycline using chitosan–alginate–bentonite composites. Appl. Clay Sci. 2023, 239, 106952. [Google Scholar] [CrossRef]
- Shahrin, E.W.E.; Narudin, N.A.H.; Shahri, N.N.M.; Nur, M.; Lim, J.-W.; Bilad, M.R.; Mahadi, A.H.; Hobley, J.; Usman, A. A comparative study of adsorption behavior of rifampicin, streptomycin, and ibuprofen contaminants from aqueous solutions onto chitosan: Dynamic interactions, kinetics, diffusions, and mechanisms. Emerg. Contam. 2023, 9, 100199. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334. [Google Scholar] [CrossRef]
- Turan, B.; Sarigol, G.; Demircivi, P. Adsorption of tetracycline antibiotics using metal and clay embedded cross-linked chitosan. Mater. Chem. Phys. 2022, 279, 125781. [Google Scholar] [CrossRef]
- Nasiri, A.; Rajabi, S.; Amiri, A.; Fattahizade, M.; Hasani, O.; Lalehzari, A.; Hashemi, M. Adsorption of tetracycline using CuCoFe2O4@Chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: Isotherm, kinetic and thermodynamic study. Arab. J. Chem. 2022, 15, 104014. [Google Scholar] [CrossRef]
- Valizadeh, K.; Bateni, A.; Sojoodi, N.; Rafiei, R.; Behroozi, A.H.; Maleki, A. Preparation and characterization of chitosan-curdlan composite magnetized by zinc ferrite for efficient adsorption of tetracycline antibiotics in water. Int. J. Biol. Macromol. 2023, 235, 123826. [Google Scholar] [CrossRef]
- Yaqubi, O.; Tai, M.H.; Mitra, D.; Gerente, C.; Neoh, K.G.; Wang, C.-H.; Andres, Y. Adsorptive removal of tetracycline and amoxicillin from aqueous solution by leached carbon black waste and chitosan-carbon composite beads. J. Environ. Chem. Eng. 2021, 9, 104988. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, W.; Zheng, Z.; Yang, Z.; Liu, Z.; Zhou, L. Fabrication and adsorption mechanism of chitosan/Zr-MOF (UiO-66) composite foams for efficient removal of ketoprofen from aqueous solution. Chem. Eng. J. 2022, 431, 134045. [Google Scholar] [CrossRef]
- Şahin, M.; Arslan, Y.; Tomul, F. Removal of naproxen and diclofenac using magnetic nanoparticles/nanocomposites. Res. Chem. Intermed. 2022, 48, 5209–5226. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Cusioli, L.F.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Caffeine removal by chitosan/activated carbon composite beads: Adsorption in tap water and synthetic hospital wastewater. Chem. Eng. Res. Des. 2022, 184, 1–12. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Effect of humic acid on pharmaceuticals adsorption using sulfonic acid grafted chitosan. J. Mol. Liq. 2017, 230, 1–5. [Google Scholar] [CrossRef]
- Davarnejad, R.; Sarvmeili, K.; Safari, Z.; Kennedy, J.F. Estrogen adsorption from an aqueous solution on the chitosan nanoparticles. Int. J. Biol. Macromol. 2023, 237, 124224. [Google Scholar] [CrossRef] [PubMed]
- Kgomo, H.; Dube, S.; Nindi, M.M. Evaluating the Performance of Ball-Milled Silk Fibroin Films for Simultaneous Adsorption of Eight Pharmaceuticals from Water. Int. J. Environ. Res. Public Health 2022, 19, 14922. [Google Scholar] [CrossRef]
- Shokri, M.; Mojtabavi, S.; Jafari-Nodoushan, H.; Vojdanitalab, K.; Golshani, S.; Jahandar, H.; Faramarzi, M.A. Laccase-loaded magnetic dialdehyde inulin nanoparticles as an efficient heterogeneous natural polymer-based biocatalyst for removal and detoxification of ofloxacin. Biodegradation 2022, 33, 489–508. [Google Scholar] [CrossRef]
- Quesada, H.B.; de Araújo, T.P.; Vareschini, D.T.; de Barros, M.A.S.D.; Gomes, R.G.; Bergamasco, R. Chitosan, alginate and other macromolecules as activated carbon immobilizing agents: A review on composite adsorbents for the removal of water contaminants. Int. J. Biol. Macromol. 2020, 164, 2535–2549. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Sattar, M.; Hayeeye, F.; Chinpa, W.; Sirichote, O. Preparation and characterization of poly (lactic acid)/activated carbon composite bead via phase inversion method and its use as adsorbent for Rhodamine B in aqueous solution. J. Environ. Chem. Eng. 2017, 5, 3780–3791. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Omer, A.M.; Hassan, M.A.; Hassan, M.E.; Sabet, M.M.; Eldin, M.S.M. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 001–006. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, Z.; Ren, R.; Sui, X.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B. Chitosan adsorbent reinforced with citric acid modified β-cyclodextrin for highly efficient removal of dyes from reactive dyeing effluents. Eur. Polym. J. 2018, 108, 212–218. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.; Hanafiah, M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef]
- Solano, R.A.; De León, L.D.; De Ávila, G.; Herrera, A.P. Polycyclic aromatic hydrocarbons (PAHs) adsorption from aqueous solution using chitosan beads modified with thiourea, TiO2 and Fe3O4 nanoparticles. Environ. Technol. Innov. 2021, 21, 101378. [Google Scholar] [CrossRef]
- Liakos, E.V.; Lazaridou, M.; Michailidou, G.; Koumentakou, I.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol 2021, 1, 130–154. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu (II)and Co (II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Biol. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Asgari, E.; Sheikhmohammadi, A.; Yeganeh, J. Application of the Fe3O4-chitosan nano-adsorbent for the adsorption of metronidazole from wastewater: Optimization, kinetic, thermodynamic and equilibrium studies. Int. J. Biol. Macromol. 2020, 164, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Ranjbari, S.; Tanhaei, B.; Ayati, A.; Orooji, Y.; Alizadeh, M.; Karimi, F.; Salmanpour, S.; Rouhi, J.; Sillanpää, M.; et al. Novel 1-butyl-3-methylimidazolium bromide impregnated chitosan hydrogel beads nanostructure as an efficient nanobio-adsorbent for cationic dye removal: Kinetic study. Environ. Res. 2021, 195, 110809. [Google Scholar] [CrossRef] [PubMed]
- Shebl, A.; Omer, A.; Tamer, T. Adsorption of cationic dye using novel O-amine functionalized chitosan Schiff base derivatives: Isotherm and kinetic studies. Desalination Water Treat. 2018, 130, 132–141. [Google Scholar] [CrossRef]
- Shankar, P.; Gomathi, T.; Vijayalakshmi, K.; Sudha, P. Comparative studies on the removal of heavy metals ions onto cross linked chitosan-g-acrylonitrile copolymer. Int. J. Biol. Macromol. 2014, 67, 180–188. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Chen, D.-Y.; Pathak, J.L.; Long, J.; Subbaiah, M.V.; Wen, J.-C.; Pan, C.-L. Preparation of novel aminated chitosan schiff’s base derivative for the removal of methyl orange dye from aqueous environment and its biological applications. Int. J. Biol. Macromol. 2020, 146, 1100–1110. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2021, 33, 100565. [Google Scholar] [CrossRef]
- Tofan, L.; Bojoaga, C.-N.; Paduraru, C. Biosorption for the recovery and analysis of rare earth elements and platinum group metals from real samples. A review. Environ. Chem. Lett. 2022, 20, 1225–1248. [Google Scholar] [CrossRef]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2018, 17, 241–257. [Google Scholar] [CrossRef]
- Brahma, B.; Das, M.; Sarkar, P.; Sarkar, U. Biosorption of p-chloro meta xylenol (PCMX) by bacterium-encapsulated calcium alginate beads in a novel plug flow process. J. Environ. Manag. 2023, 337, 117764. [Google Scholar] [CrossRef] [PubMed]






| Therapeutic Group | Ecological and Human Health Effects | Reference |
|---|---|---|
| Antibiotics | Limit the therapeutic effectiveness of antibiotics used to treat human and animal infections by causing the development of antibiotic-resistant bacteria in the environment. Affects the growth of cyanobacteria and green algae, as well as leads to the development of antibiotic resistance in microorganisms. Acute and chronic exposure causes histopathological changes in some fish species | [5] |
| Analgesics | Increased production of antioxidant enzymes in mollusks (Dreissena polymorpha). Generate higher oxidative stress in fishes. Human exposure has been linked to serious health problems such as liver damage, myocardial infarction, nephrotoxicity, hypertension, cerebrovascular accidents, gastrointestinal bleeding, and fetal development impairment. | [16,17,18,19] |
| Anti-inflammatory | Can cause tissue damage to aquatic communities, affecting their growth and metabolism (i.e., alterations in gills and renal lesions at fish). | [5] |
| Endocrine disruptors | Human exposure can cause changes in the reproductively relevant, sexually dimorphic neuroendocrine system, alterations in endogenous steroid levels, diabetes, cardiovascular problems, aberrant neural behaviors, and is associated to obesity. | [20] |
| P (Problem) | Presence of pharmaceutical compounds as emergent pollutants in different water sources |
| I (Intervention) | Applying innovative biomaterials based on microbial biomass and natural polymers to remove pharmaceuticals from aqueous matrices. |
| C (Comparison) | Biomaterials such as microbial cells, residual microbial biomass, natural polymers and biocomposites used in pharmaceutical compound removal processes |
| O (Outcome(s)) | Improving pharmaceutical removal methods by identifying viable and sustainable materials to enable large-scale application of biosorption technologies. |
| Biosorbent | Therapeutic Group/ Pharmaceutical Compound | Process Parameters | Obtained Results | Ref. |
|---|---|---|---|---|
| ALGINATE—BASE FOR IMMOBILIZATION/ENCAPSULATION | ||||
| Antibiotics | ||||
| Saccharomyces cerevisiae/calcium alginate composite beads (inactive cells) | Cephalexin | pH = 2 ÷ 12; temperature = 25 °C; initial concentration of the pharmaceutical compound = 10–80 mg/L; biosorbent dose = 0.5 ÷ 3 g/L; time = 12 h; batch system | Removal efficiency was 86.23% for C0 = 30 mg/L; pH = 4; biosorbent dose = 1 g/L and the biosorption capacity was 94.34 mg/g | [48] |
| Saccharomyces pastorianus residual microbial biomass/calcium alginate composite beads Immobilization technique (inactive cells) | Cephalexin | pH = 5; initial concentration of the pharmaceutical compound = 10–80 mg/L; biosorbent dose = 0.5 ÷ 3 g/L; time = 12 h; room temperature; batch system | Biosorption capacity = 9.26 mg/g | [52] |
| Antiseptics | ||||
| Saccharomyces cerevisiae/calcium alginate composite beads (inactive cells) | Ethacridine lactate | pH = 5; initial concentration of the pharmaceutical compound = 100 mg/L; biosorbent beads dose = 1 g/25 mL drug solution; time = 12 h; room temperature; batch system | Removal efficiency was 96.40% | [52] |
| Saccharomyces pastorianus/ calcium alginate composite beads (inactive cells) | Ethacridine lactate | pH = 2–10; initial concentration of the pharmaceutical compound = 20–60 mg/L; biosorbent beads dose = 1–3 g/L; time = 24 h; room temperature; batch system | Biosorption capacity was 26.72 mg/g and the removal efficiency was 91.05% for C0 = 60 mg/L; pH = 4; biosorbent dose = 2 g/L | [49] |
| Saccharomyces pastorianus residual microbial biomass/calcium alginate composite beads Immobilization technique (inactive cells) | Ethacridine lactate | Biosorption capacity was 26.76 mg/g and the removal efficiency was 89.93% for C0 = 60 mg/L; pH = 4; biosorbent dose = 2 g/L | [49] | |
| Saccharomyces pastorianus residual biomass / calcium alginate composite beads Encapsulation technique (inactive cells) | Ethacridine lactate | pH = 2–10; initial concentration of the pharmaceutical compound = 20–60 mg/L; biosorbent beads dose = 1–3 g/L; time = 24 h; room temperature; batch system | Biosorption capacity was 21.39 mg/g and the removal efficiency was 85% for C0 = 50 mg/L; pH = 2; biosorbent dose = 2 g/L | [15] |
| Pandoraea sp. strain BT102 (bacterium-encapsulated in calcium alginate beads) (active cells) | p-Chloro-meta- xylenol | pH = 10 ± 0.2.; temperature = 37 °C; initial concentration of the pharmaceutical compound = 1–100 mg/L; biosorbent beads dose = 100 g/L of drug solution; time = 16 h; shaker at a rotating speed of 120 rpm; batch system | Maximum adsorption capacity was 961.7 mg/g for C0 = 100 mg/L after 4 h | [137] |
| CHITOSAN—BASE FOR IMMOBILIZATION/ENCAPSULATION | ||||
| Antibiotics | ||||
| Saccharomyces cerevisiae/ chitosan composite beads (inactive cells) | Cephalexin | pH = 5–6; initial concentration of the pharmaceutical compound = 30–50 mg/L; biosorbent beads dose = 1 g/25 mL of drug solution; time = 12 h; room temperature; batch system | Maximum adsorption capacity was 22.78 mg/g for C0= 30 mg/L and pH = 5 | [52] |
| Rifampicin | Maximum adsorption capacity was 24.70 mg/g for C0= 50 mg/L and pH = 6 | |||
| Saccharomyces pastorianus/ chitosan composite beads (inactive cells) | Cephalexin | pH = 5–6; initial concentration of the pharmaceutical compound = 30–50 mg/L; biosorbent beads dose = 1 g/25 mL of drug solution; time = 12 h; room temperature; batch system | Maximum adsorption capacity was 28.42 mg/g for C0= 30 mg/L and pH = 5 | [52] |
| Rifampicin | Maximum adsorption capacity was 24.89 mg/g for C0= 50 mg/L and pH = 6 | |||
| OTHER MATERIALS USED FOR IMMOBILIZATION/ENCAPSULATION | ||||
| Anti-epileptics | ||||
| Phanerochaete chrysosporium immobilized in wood chips (inactive cells) | Carbamazepine | pH =3.2 ÷ 4.5; temperature = 30 °C; the pharmaceutical compound added into the influent at 1.0 mg/L; time = 28 days; aerating mode; fixed-bed bioreactor | Removal efficiency on day 7, in coexistence with naproxen, was 61.30 ± 3.84% and biosorption capacity was 0.06 mg/g | [84] |
| Anti-inflammatories | ||||
| Phanerochaete chrysosporium immobilized in wood chips (inactive cells) | Naproxen | pH =3.2 ÷ 4.5; temperature = 30 °C; the pharmaceutical compound added into the influent at 1.0 mg/L; time = 28 days; aerating mode; fixed-bed bioreactor | Removal efficiency on day 7 in coexistence with carbamazepine was 90.35 ± 4.37% and biosorption capacity was 0.17 mg/g | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, L.; Suceveanu, E.-M.; Blaga, A.-C.; Nedeff, F.M.; Șuteu, D. Insights into Recent Advances of Biomaterials Based on Microbial Biomass and Natural Polymers for Sustainable Removal of Pharmaceuticals Residues. Polymers 2023, 15, 2923. https://doi.org/10.3390/polym15132923
Rusu L, Suceveanu E-M, Blaga A-C, Nedeff FM, Șuteu D. Insights into Recent Advances of Biomaterials Based on Microbial Biomass and Natural Polymers for Sustainable Removal of Pharmaceuticals Residues. Polymers. 2023; 15(13):2923. https://doi.org/10.3390/polym15132923
Chicago/Turabian StyleRusu, Lăcrămioara, Elena-Mirela Suceveanu, Alexandra-Cristina Blaga, Florin Marian Nedeff, and Daniela Șuteu. 2023. "Insights into Recent Advances of Biomaterials Based on Microbial Biomass and Natural Polymers for Sustainable Removal of Pharmaceuticals Residues" Polymers 15, no. 13: 2923. https://doi.org/10.3390/polym15132923