Influence of Reduction with NaBH4 and HCl in Obtaining Amino Derivatives of Cashew Gum and Cytotoxic Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materiais
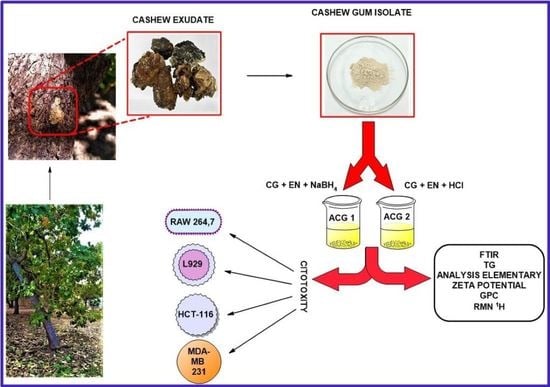
2.2. Purification of the Cashew Gum
2.3. Modification Reaction
2.4. Characterization of Cashew Gum Amine
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Elemental Analysis(C, H, N)
2.4.4. Degree of Substitution
2.4.5. Zeta Potential
2.4.6. Gel Permeation Chromatography (GPC)
2.4.7. Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.4.8. Cytotoxicity
Cell Lines and Cell Culture
MTT Assay
3. Results
3.1. Fourier Transform Infrared Spectroscopy
3.2. Thermogravimetric Analysis (TG)
3.3. Elementary Analysis (C, H, N)
3.4. Zeta Potential
3.5. Gel Permeation Chromatography
3.6. Hydrogen Magnetic Resonance (NMR 1H)
3.7. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amiri, M.S.; Mohammadzadeh, V.; Ehsan, M.; Yazdi, T. Plant-Based Gums and Mucilages Applications in Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-krok, E. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural Gum-Based Functional Bioactive Films and Coatings: A Review. Sci. Mol. 2023, 24, 485. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Ribeiro, J.; Ribeiro, A.B.; Freitas, A.R.; Del, M.; Collado-gonzalez, M.; Silva, L.R.; Alves, L.; Melro, E.; Antunes, F.E.; Veiga, F.; et al. Modification of chicha gum: Antibacterial activity, ex vivo mucoadhesion, antioxidant activity and cellular viability. Int. J. Biol. Macromol. 2023, 228, 594–603. [Google Scholar]
- Yamassaki, F.T.; Lenzi, R.M.; Campestrini, L.H.; Bovo, F.; Seyfried, M.; Soldera-Silva, A.; Stevan-Hancke, F.R.; Zawadzki-Baggio, S.F.; Pettolino, F.A.; Bacic, A.; et al. Effect of the native polysaccharide of cashew-nut tree gum exudate on murine peritoneal macrophage modulatory activities. Carbohydr. Polym. 2015, 125, 241–248. [Google Scholar] [CrossRef]
- Araújo, T.S.L.; Costa, D.S.; Sousa, N.A.; Souza, L.K.M.; De Araújo, S.; Oliveira, A.P.; Sousa, F.B.M.; Silva, D.A.; Barbosa, A.L.R.; Leite, J.R.S.A.; et al. Antidiarrheal activity of cashew GUM, a complex heteropolysaccharide extracted from exudate of Anacardium occidentale L. in rodents. J. Ethnopharmacol. 2015, 174, 299–307. [Google Scholar] [CrossRef]
- Pitombeira, N.A.O.; Veras Neto, J.G.; Silva, D.A.; Feitosa, J.P.A.; Paula, H.C.B.; De Paula, R.C.M. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum arabic: A review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Phillips, G.O. Metabolism and calorific value Acacia gum (Gum Arabic): A nutritional fibre; metabolism and calorific value. Food Addit. Contam. 1998, 15, 37–41. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Tambourgi, E.B. Goma Do Cajueiro Exacta. Exacta 2007, 5, 145–154. [Google Scholar]
- Schirato, G.V.; Monteiro, F.M.F.; Silva, F.d.O.; Lima Filho, J.L.d.; Leão, A.M.d.A.C.; Porto, A.L.F. O polissacarídeo do Anacardium occidentale L. na fase inflamatória do processo cicatricial de lesões cutâneas. Ciência Rural 2006, 36, 149–154. [Google Scholar] [CrossRef]
- Campos, D.A.; Ribeiro, A.C.; Costa, E.M.; Fernandes, J.C.; Tavaria, F.K.; Araruna, F.B.; Eiras, C.; Eaton, P.; Leite, J.R.S.A.; Manuela Pintado, M. Study of antimicrobial activity and atomic force microscopy imaging of the action mechanism of cashew tree gum. Carbohydr. Polym. 2012, 90, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Quelemes, P.V.; de Araújo, A.R.; Plácido, A.; Delerue-Matos, C.; Maciel, J.S.; Bessa, L.J.; Ombredane, A.S.; Joanitti, G.A.; Soares, M.J.d.S.; Eaton, P.; et al. Quaternized cashew gum: An anti-staphylococcal and biocompatible cationic polymer for biotechnological applications. Carbohydr. Polym. 2017, 157, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, W.J.; Batista, K.D.A. Uso de Goma de Cajueiro em Substituição ao Ágar em Meio de Cultura. Rev. Biotecnol. Ciência 2012, 2, 1–15. [Google Scholar]
- Andrade, K.C.S.; De Carvalho, C.W.P.; Takeiti, C.Y.; De Azeredo, H.M.C.; Corrêa, J.D.S.; Caldas, C.M. Goma de Cajueiro (Anacardium occidentale): Avaliação das Modificações Químicas e Físicas por Extrusão Termoplástica. Polimeros 2013, 23, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.A.; De Paula, R.C.M.; Feitosa, J.P.A.; De Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of cashew tree exudate polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Cunha, P.L.R.; Maciel, J.S.; Sierakowski, M.R.; De Paulaa, R.C.M.; Feitosa, J.P.A. Oxidation of cashew tree gum exudate polysaccharide with TEMPO reagent. J. Braz. Chem. Soc. 2007, 18, 85–92. [Google Scholar] [CrossRef] [Green Version]
- De Pinto, G.L.; Martinez, M.; Mendoza, J.A.; Ocando, E.; Rivas, C. Comparison of three anacardiaceae gum exudates. Biochem. Syst. Ecol. 1995, 23, 151–156. [Google Scholar] [CrossRef]
- Porto, B.C.; Augusto, P.E.D.; Cristianini, M. A Comparative Study Between Technological Properties of Cashew Tree Gum and Arabic Gum. J. Polym. Environ. 2015, 23, 392–399. [Google Scholar] [CrossRef]
- Paula, H.C.B.; Rodrigues, M.L.L.; Ribeiro, W.L.C.; Stadler, A.S.; Paula, R.C.M.; Abreu, F.O.M.S. Protective Effect of Cashew Gum Nanoparticles on Natural Larvicide from Moringa oleifera Seeds. J. Appl. Polym. Sci. 2011, 124, 1178–1784. [Google Scholar] [CrossRef]
- Carvalho, N.S.; Silva, M.M.; Silva, R.O.; Nicolau, L.A.D.; Sousa, F.B.M.; Damasceno, S.R.B.; Silva, D.A.; Barbosa, A.L.R.; Leite, J.R.S.A.; Medeiros, J.V.R. Gastroprotective properties of cashew gum, a complex heteropolysaccharide of Anacardium occidentale, in naproxen-induced gastrointestinal damage in rats. Drug Dev. Res. 2015, 76, 143–151. [Google Scholar] [CrossRef]
- De Moura Neto, É.; Da, S.; Maciel, J.; Cunha, P.L.R.; De Paula, R.C.M.; Feitosa, J.P.A. Preparation and characterization of a chemically sulfated cashew gum polysaccharide. J. Braz. Chem. Soc. 2011, 22, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Simi, C.K.; Abraham, T.E. Physico chemical properties of aminated tamarind xyloglucan. Colloids Surf. B Biointerfaces 2010, 81, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, N.; Palanisamy, M.; Jeyaraj, W.; Karuppasamy, G. Stable and robust nanobiocomposite preparation using aminated guar gum (mimic activity of graphene) with electron beam irradiated polypyrrole and Ce-Ni bimetal: Effective role in simultaneous sensing of environmental pollutants and pseudocapacitor applications. Electrochim. Acta 2017, 246, 484–496. [Google Scholar] [CrossRef]
- Marcelino, M.M.; Vieira de Melo, S.A.B.; Torres, E.A. Caracterização da biomassa da casca de coco para obtenção de energia. BA&D 2017, 27, 337–355. [Google Scholar]
- Hani, U.; Shivakumar, H.G. Design and optimization of clotrimazole–hydroxypropyl-b-cyclodextrin bioadhesive vaginal tablets using Anacardium occidentale gum by 32 factorial design. RSC Adv. 2015, 5, 35391–35404. [Google Scholar] [CrossRef]
- De Paula, R.C.M.; Santana, S.A.; Rodrigues, J.F. Composition and rheological properties of Albizia lebbeck gum exudate. Carbohydr. Polym. 2001, 44, 133–139. [Google Scholar] [CrossRef]
- Yun, J.C.; Lee, S.; Mi, A.Y.; Hyeon, G.L. Structural and biological characterization of sulfated-derivatized oat β-glucan. J. Agric. Food Chem. 2006, 54, 3815–3818. [Google Scholar]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Mothé, C.G.; Carestiato, T.; Silva, S.P.S.; Aguila, M.B. Thermoanalytical study of organs of spontaneous hypertension rats. J. Therm. Anal. Calorim. 2006, 85, 61–63. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Neamtu, I.; Bercea, M. Study of a binary interpenetrated polymeric complex by correlation of rheological parameters with zeta potential and conductivity. Colloids Surf. B Biointerfaces 2010, 76, 70–75. [Google Scholar] [CrossRef]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Silva, D.A.; Feitosa, J.P.A.; Maciel, J.S.; Paula, H.C.B.; de Paula, R.C.M. Characterization of crosslinked cashew gum derivatives. Carbohydr. Polym. 2006, 66, 16–26. [Google Scholar] [CrossRef]
- Mothé, C.G.; Rao, M.A. Thermal behavior of gum arabic in comparison with cashew gum. Thermochim. Acta 2000, 357–358, 9–13. [Google Scholar] [CrossRef]
- Peniche-Covas, C.; Argüelles-Monal, W.; San Román, J. A kinetic study of the thermal degradation of chitosan and a mercaptan derivative of chitosan. Polym. Degrad. Stab. 1993, 39, 21–28. [Google Scholar] [CrossRef]
- Varma, A.J.; Kokane, S.P.; Pathak, G.; Pradhan, S.D. Thermal behavior of galactomannan guar gum and its periodate oxidation products. Carbohydr. Polym. 1997, 32, 111–114. [Google Scholar] [CrossRef]
- Freitas, R.A.; Martin, S.; Paula, R.C.; Feitosa, J.P.A.; Sierakowskid, M.R. Effect of the oxidation level on the thermogravimetric kinetics of an oxidized galactoxyloglucan from Hymenaea courbaril (Jatobá) seeds. Thermochim. Acta 2004, 409, 41–47. [Google Scholar] [CrossRef]
- Richter, A.R.; Carneiro, M.J.; de Sousa, N.A.; Pinto, V.P.T.; Freire, R.S.; de Sousa, J.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Feitosa, J.P.A.; Paula, H.C.B.; et al. Self-assembling cashew gum-graft-polylactide copolymer nanoparticles as a potential amphotericin B delivery matrix. Int. J. Biol. Macromol. 2020, 152, 492–502. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohydr. Polym. 2010, 80, 1172–1177. [Google Scholar] [CrossRef]
- Li, B.; Dong, M.; De, J.; Ye, L.; Chen, D.; Lu, Y. Structural characterization and anti-proliferation activities against tumor cells of an arabinogalactan from Juniperus convallium. Molecules 2019, 24, 1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, A.B.; Moura, A.F.; Silva, D.A.; Oliveira, T.M.; Barreto, F.S.; Ribeiro, W.L.C.; Alves, A.P.N.N.; Araújo, A.J.; Moraes Filho, M.O.; Iles, B.; et al. Evaluation of antitumor potential of cashew gum extracted from Anacardium occidentale Linn. Int. J. Biol. Macromol. 2020, 154, 319–328. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.K.A.; Ribeiro, F.O.S.; de Oliveira, T.M.; de Araújo, A.R.; Dias, J.D.N.; Albuquerque, P.; Silva-Pereira, I.; de Jesus Oliveira, A.C.; Quelemes, P.V.; Leite, J.R.S.A.; et al. Quaternization of angico gum and evaluation of anti-staphylococcal effect and toxicity of their derivatives. Int. J. Biol. Macromol. 2020, 150, 1175–1183. [Google Scholar] [CrossRef] [PubMed]






| Data | CG | CGA1 | CGA2 |
|---|---|---|---|
| Tinitial a (°C) | 247 | 192 | 215 |
| Tinitial b (°C) | 473 | 531 | 531 |
| Tmáx (°C) in the range | |||
| 220–260 | 248 (sh) | 189 (I) | - |
| 260–350 | 310 (II) | 270 (II) | 287 (I) |
| 400–450 | 428 (III) | - | - |
| 450–600 | 473 (IV) | 532 (III) | 530 (II) |
| Residual mass 800 °C (%) | 1.7 | 1.7 | 1.7 |
| CG | CGA1 | CGA2 | ||||
|---|---|---|---|---|---|---|
| Moisture | 6.7 | 5.6 | 5.6 | |||
| Ashes | 1.7 | 1.8 | 1.1 | |||
| pH | 7.43 | 7.53 | 7.50 | |||
| Zeta | −26.5 | +0.16 | −3.68 | |||
| Elementary analysis | %C | 36.06 | %C | 32.12 | %C | 36.29 |
| %H | 5.68 | %H | 6.01 | %H | 5.24 | |
| %N | 0.76 | %N | 8.73 | %N | 2.74 | |
| Protein | 0.15 | 1.72 | 0.54 | |||
| DS | - | 1.09 | 0.32 | |||
| Mpk (g/mol) | 2.29 × 104 | 7.83 × 103 | 1.25 ×104 | |||
| Mn (g/mol) | 4.00 × 103 | 1.07 ×103 | 3.04 ×103 | |||
| MW (g/mol) | 2.12 × 104 | 6.56 × 104 | 1.10 × 104 | |||
| IPD | 5.3 | 6.1 | 3.6 | |||
| Sample | IC50 (mg/mL) Confidence Interval 95% | |||
|---|---|---|---|---|
| MDA-MB-231 | HCT-116 | L929 | RAW 264.7 | |
| ACG1 | 3.2 (2.5–3.9) | 4.1 (3.5–4.6) | 6.9 (6.0–7.9) | >50 |
| ACG2 | 4.0 (3.2–5.0) | 2.4 (2.2–2.6) | 3.9 (3.3–4.5) | >50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, F.d.C.M.; Lopes, W.C.; Ribeiro, F.O.S.; Rodrigues, R.R.L.; França Rodrigues, K.A.d.; Santos Machado, F.d.; Araújo, A.J.; Marinho Filho, J.D.B.; Oliveira, A.C.J.; Filho, E.C.S.; et al. Influence of Reduction with NaBH4 and HCl in Obtaining Amino Derivatives of Cashew Gum and Cytotoxic Profile. Polymers 2023, 15, 2856. https://doi.org/10.3390/polym15132856
Brito FdCM, Lopes WC, Ribeiro FOS, Rodrigues RRL, França Rodrigues KAd, Santos Machado Fd, Araújo AJ, Marinho Filho JDB, Oliveira ACJ, Filho ECS, et al. Influence of Reduction with NaBH4 and HCl in Obtaining Amino Derivatives of Cashew Gum and Cytotoxic Profile. Polymers. 2023; 15(13):2856. https://doi.org/10.3390/polym15132856
Chicago/Turabian StyleBrito, Francisco das C. M., Wilton C. Lopes, Fábio O. S. Ribeiro, Raiza Raianne Luz Rodrigues, Klinger Antonio da França Rodrigues, Fabrício dos Santos Machado, Ana Jérsia Araújo, José Delano Barreto Marinho Filho, Antônia Carla J. Oliveira, Edson C. S. Filho, and et al. 2023. "Influence of Reduction with NaBH4 and HCl in Obtaining Amino Derivatives of Cashew Gum and Cytotoxic Profile" Polymers 15, no. 13: 2856. https://doi.org/10.3390/polym15132856