Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Phase Diagrams Definition and Emulsification Process
2.3. Emulsion Type Determination
2.4. Yield of Oil Incorporation and Oil Release during Storage
2.5. Droplet Size Measurement
2.6. PEs Stability
2.7. Contact Angle Measurements
2.8. Rheological Measurements
2.9. Statistical Analysis
3. Results
3.1. PEs Formation
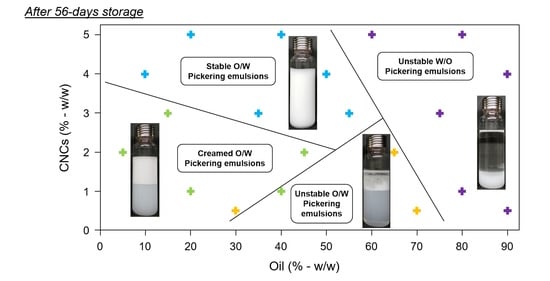
3.2. PEs Stability
3.3. Study of Mechanisms Acting on O/W PEs-I Formation and Stability
3.4. Hypothesis of Mechanisms Involved in PEs Formation and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chappat, M. Some Applications of Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 57–77. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef] [Green Version]
- Seweryn, A. Interactions between Surfactants and the Skin—Theory and Practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of Food Additives on the Composition and Function of Gut Microbiota: A Review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food Additive Emulsifiers: A Review of Their Role in Foods, Legislation and Classifications, Presence in Food Supply, Dietary Exposure, and Safety Assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, Properties, Applications, Trends, and General Perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-Based Pickering Emulsions: Formation, Stabilization and Applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Gauthier, G.; Capron, I. Pickering Nanoemulsions: An Overview of Manufacturing Processes, Formulations, and Applications. JCIS Open 2021, 4, 100036. [Google Scholar] [CrossRef]
- Ming, Y.; Xia, Y.; Ma, G. Aggregating Particles on the O/W Interface: Tuning Pickering Emulsion for the Enhanced Drug Delivery Systems. Aggregate 2022, 3, e162. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Li, D.S.; Ilavsky, J.; Kuzmenko, I.; Jeng, G.-S.; O’Donnell, M.; Pozzo, L.D. Ultrasound-Based Formation of Nano-Pickering Emulsions Investigated via in-Situ SAXS. J. Colloid Interface Sci. 2019, 536, 281–290. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.; Huang, Q. Recent Advances on Food-Grade Particles Stabilized Pickering Emulsions: Fabrication, Characterization and Research Trends. Trends Food Sci. Technol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.-H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Niroula, A.; Gamot, T.D.; Ooi, C.W.; Dhital, S. Biomolecule-Based Pickering Food Emulsions: Intrinsic Components of Food Matrix, Recent Trends and Prospects. Food Hydrocoll. 2021, 112, 106303. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering Emulsions for Sustainable Micro/Nanocellulose in Food and Cosmetic Applications. Polymers 2020, 12, 2385. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different Preparation Methods and Properties of Nanostructured Cellulose from Various Natural Resources and Residues: A Review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.; Fang, R.; Lei, C.; Li, Y.; Li, B.; Pei, Y.; Luo, X. Application of Nanocellulose as Particle Stabilizer in Food Pickering Emulsion: Scope, Merits and Challenges. Trends Food Sci. Technol. 2021, 110, 573–583. [Google Scholar] [CrossRef]
- Pang, B.; Liu, H.; Zhang, K. Recent Progress on Pickering Emulsions Stabilized by Polysaccharides-Based Micro/Nanoparticles. Adv. Colloid Interface Sci. 2021, 296, 102522. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, N.; Fathi, M. Production of Cellulose Nanocrystals from Pistachio Shells and Their Application for Stabilizing Pickering Emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Gong, J.; Kuang, Y.; Mo, L.; Song, T. Cellulose Nanocrystals (CNCs) with Different Crystalline Allomorph for Oil in Water Pickering Emulsions. Carbohydr. Polym. 2018, 183, 303–310. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic Nanorods of Various Aspect Ratios for Oil in Water Pickering Emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Shin, J.; Na, K.; Shin, S.; Seo, S.-M.; Youn, H.J.; Park, I.-K.; Hyun, J. Biological Activity of Thyme White Essential Oil Stabilized by Cellulose Nanocrystals. Biomolecules 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Li, J.; Fan, L. Production of Nanocellulose with Different Length from Ginkgo Seed Shells and Applications for Oil in Water Pickering Emulsions. Int. J. Biol. Macromol. 2020, 149, 617–626. [Google Scholar] [CrossRef]
- Hedjazi, S.; Razavi, S.H. A Comparison of Canthaxanthine Pickering Emulsions, Stabilized with Cellulose Nanocrystals of Different Origins. Int. J. Biol. Macromol. 2018, 106, 489–497. [Google Scholar] [CrossRef]
- Meirelles, A.A.D.; Costa, A.L.R.; Cunha, R.L. Cellulose Nanocrystals from Ultrasound Process Stabilizing O/W Pickering Emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef]
- Kinra, S.; Pal, R. Rheology of Pickering Emulsions Stabilized and Thickened by Cellulose Nanocrystals over Broad Ranges of Oil and Nanocrystal Concentrations. Colloids Interfaces 2023, 7, 36. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-Water Pickering Emulsions via Microfluidization with Cellulose Nanocrystals: 1. Formation and Stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Varanasi, S.; Henzel, L.; Mendoza, L.; Prathapan, R.; Batchelor, W.; Tabor, R.; Garnier, G. Pickering Emulsions Electrostatically Stabilized by Cellulose Nanocrystals. Front. Chem. 2018, 6, 409. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and Stabilization of D-Limonene Pickering Emulsions by Cellulose Nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef]
- Beck, S.; Méthot, M.; Bouchard, J. General Procedure for Determining Cellulose Nanocrystal Sulfate Half-Ester Content by Conductometric Titration. Cellulose 2015, 22, 101–116. [Google Scholar] [CrossRef]
- Qian, X.; Lu, Y.; Ge, L.; Yin, S.; Wu, D. Starch Nanocrystals as the Particle Emulsifier to Stabilize Caprylic/Capric Triglycerides-in-Water Emulsions. Carbohydr. Polym. 2020, 245, 116561. [Google Scholar] [CrossRef]
- Dziza, K.; Santini, E.; Liggieri, L.; Jarek, E.; Krzan, M.; Fischer, T.; Ravera, F. Interfacial Properties and Emulsification of Biocompatible Liquid-Liquid Systems. Coatings 2020, 10, 397. [Google Scholar] [CrossRef]
- Asfour, M.H.; Elmotasem, H.; Mostafa, D.M.; Salama, A.A.A. Chitosan Based Pickering Emulsion as a Promising Approach for Topical Application of Rutin in a Solubilized Form Intended for Wound Healing: In Vitro and In Vivo Study. Int. J. Pharm. 2017, 534, 325–338. [Google Scholar] [CrossRef]
- Capron, I.; Cathala, B. Surfactant-Free High Internal Phase Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2013, 14, 291–296. [Google Scholar] [CrossRef]
- Miao, C.; Tayebi, M.; Hamad, W.Y. Investigation of the Formation Mechanisms in High Internal Phase Pickering Emulsions Stabilized by Cellulose Nanocrystals. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170039. [Google Scholar] [CrossRef] [Green Version]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The Theory of Emulsification1. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, X.; Chang, S.; Ou, W.; Zhang, W. Effect of Oil Properties on the Formation and Stability of Pickering Emulsions Stabilized by Ultrafine Pearl Powder. J. Mol. Liq. 2021, 338, 116645. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Liu, T.-X.; Tang, C.-H. Tuning the Stability and Microstructure of Fine Pickering Emulsions Stabilized by Cellulose Nanocrystals. Ind. Crops Prod. 2019, 141, 111733. [Google Scholar] [CrossRef]
- Ataeian, P.; Aroyan, L.; Parwez, W.; Tam, K.C. Emulsions Undergoing Phase Transition: Effect of Emulsifier Type and Concentration. J. Colloid Interface Sci. 2022, 617, 214–223. [Google Scholar] [CrossRef]
- Arditty, S.; Whitby, C.P.; Binks, B.P.; Schmitt, V.; Leal-Calderon, F. Some General Features of Limited Coalescence in Solid-Stabilized Emulsions. Eur. Phys. J. E 2003, 11, 273–281. [Google Scholar] [CrossRef]
- Ridel, L.; Bolzinger, M.-A.; Gilon-Delepine, N.; Dugas, P.-Y.; Chevalier, Y. Pickering Emulsions Stabilized by Charged Nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Chen, X.; McClements, D.J.; Zou, L.; Liu, W. Pickering-Stabilized Emulsion Gels Fabricated from Wheat Protein Nanoparticles: Effect of PH, NaCl and Oil Content. J. Dispers. Sci. Technol. 2018, 39, 826–835. [Google Scholar] [CrossRef]
- Pandey, A.; Derakhshandeh, M.; Kedzior, S.A.; Pilapil, B.; Shomrat, N.; Segal-Peretz, T.; Bryant, S.L.; Trifkovic, M. Role of Interparticle Interactions on Microstructural and Rheological Properties of Cellulose Nanocrystal Stabilized Emulsions. J. Colloid Interface Sci. 2018, 532, 808–818. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrin, L.; Desobry-Banon, S.; Gillet, G.; Desobry, S. Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Polymers 2023, 15, 2783. https://doi.org/10.3390/polym15132783
Perrin L, Desobry-Banon S, Gillet G, Desobry S. Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Polymers. 2023; 15(13):2783. https://doi.org/10.3390/polym15132783
Chicago/Turabian StylePerrin, Louise, Sylvie Desobry-Banon, Guillaume Gillet, and Stephane Desobry. 2023. "Phase Diagram of Pickering Emulsions Stabilized by Cellulose Nanocrystals" Polymers 15, no. 13: 2783. https://doi.org/10.3390/polym15132783