Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
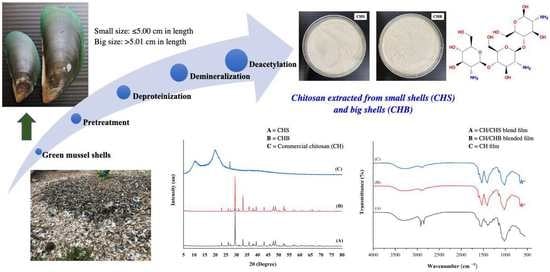
2.3. Extraction of Chitosan from Green Mussel Shells
2.4. Characterization of Chitosan
2.4.1. Chitosan Appearance and Color
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis and Determination of Degree of Deacetylation
2.4.3. Solubility
2.4.4. Determination of Water-Binding Capacity
2.4.5. Determination of Fat-Binding Capacity
2.4.6. X-ray Diffraction (XRD) Analysis
2.5. Preparation of Extracted Chitosan/Commercial Chitosan Blended Films
2.6. Characterization of Chitosan Blended Films
2.6.1. Film Thickness
2.6.2. Mechanical Properties
2.6.3. Films Appearance and Color
2.6.4. Water Vapor Permeability (WVP)
2.6.5. Fourier Transform Infrared (FTIR) Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Green Mussel Shells
3.2. Characterization of Chitosan Extracted from Green Mussel Shells
3.2.1. Visual Appearance and Color Attributes of Chitosan Powders
3.2.2. The Percentage of Yield and Degree of Deacetylation (DDA)
3.2.3. Solubility
3.2.4. Water-Binding Capacity and Fat-Binding Capacity
3.2.5. FTIR Analysis
3.2.6. XRD Analysis
3.3. Characterization of Chitosan Blended Films
3.3.1. Film Thickness and Mechanical Properties
3.3.2. Visual Appearance and Color Analysis of Chitosan Blended Films
3.3.3. Water Vapor Permeability (WVP)
3.3.4. Fourier Transform Infrared (FTIR) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics of Marine Shellfish Culture Survey. 2021. Available online: https://www4.fisheries.go.th/local/file_document/20220721143342_1_file.pdf (accessed on 3 May 2023).
- Pratama, G.; Munandar, A.; Surilayani, D.; Rizky, J.A.; Hasanah, A.N.; Haryati, S.; Meata, B.A.; Nuryadin, D.F.E.; Aditia, R.P. Characteristics of crab shells and green mussel shells as potential chitosan material from Karangantu, Banten, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1137, 012033. [Google Scholar] [CrossRef]
- Cadano, J.R.; Jose, M.; Lubi, A.G.; Maling, J.N.; Moraga, J.S.; Shi, Q.Y.; Vegafria, H.M.; VinceCruz-Abeledo, C.C. A comparative study on the raw chitin and chitosan yields of common bio-waste from Philippine seafood. Environ. Sci. Pollut. Res. 2021, 28, 11954–11961. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, chitosan, and nanochitin: Extraction, synthesis, and applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Hosney, A.; Ullah, S.; Barčauskaitė, K. A review of the chemical extraction of chitosan from shrimp wastes and prediction of factors affecting chitosan yield by using an artificial neural network. Mar. Drugs 2022, 20, 675. [Google Scholar] [CrossRef]
- da Silva Lucas, A.J.; Oreste, E.Q.; Costa, H.L.G.; López, H.M.; Saad, C.D.M.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Kaewprachu, P.; Jaisan, C.; Senphan, T.; Nagarajan, M.; Wangtueai, S. Extraction and physico–chemical characterization of chitosan from mantis shrimp (Oratosquilla nepa) shell and the development of bio-composite film with agarose. Polymers 2022, 14, 3983. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Ultrasound-assisted extraction of chitosan from squid pen: Molecular characterization and fat binding capacity. J. Food. Sci. 2019, 84, 224–234. [Google Scholar] [CrossRef]
- Sudatta, B.P.; Sugumar, V.; Varma, R.; Nigariga, P. Extraction, characterization and antimicrobial activity of chitosan from pen shell, Pinna bicolor. Int. J. Biol. Macromol. 2020, 163, 423–430. [Google Scholar] [CrossRef]
- Varma, R.; Vasudevan, S. Extraction, characterization, and antimicrobial activity of chitosan from horse mussel modiolus modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and characterization of chitosan-fungal extract films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Ismail, M.; El-Feky, A.R.; Zaghloul, E.; Madkour, F.F.; Elnemr, A.; Ibrahim, H.A. Extraction of chitosan from Aristeus antennatus shells as a prior for biodegradable plastic production. Alfarama J. Basic Appl. Sci. 2023, 4, 155–170. [Google Scholar] [CrossRef]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Shojaosadati, S.A.; Dorkoosh, F.A.; Elsabee, M.Z. Enhancement and characterization of chitosan extraction from the wastes of shrimp packaging plants. J. Polym. Environ. 2011, 19, 776–783. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- ASTM. ASTM books of standards. In Standard Test Method for Water Vapor Transmission of Materials E96–80; American Society for Testing and Materials: West Conshohocken, PA, USA, 1980. [Google Scholar]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Chakraborty, A.; Parveen, S.; Chanda, D.K.; Aditya, G. An insight into the structure, composition and hardness of a biological material: The shell of freshwater mussels. RSC Adv. 2020, 10, 29543–29554. [Google Scholar] [CrossRef] [PubMed]
- Smithmaitrie, R.; Salaenoi, J.; Boonprab, K. Pigment profiles in meat and calcium carbonate content deposited in green mussel (Perna viridis). Thai Sci. Technol. J. 2019, 27, 706–716. [Google Scholar]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT-Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H. Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeorohita): Extraction and characterization. Afr. J. Biotechnol. 2016, 15, 1258–1268. [Google Scholar]
- Hossain, M.S.; Iqbal, A. Production and characterization of chitosan from shrimp waste. J. Bangladesh Agric. Univ. 2014, 12, 153–160. [Google Scholar] [CrossRef]
- Iber, B.T.; Torsabo, D.; Chik, C.E.N.C.E.; Wahab, F.; Sheikh Abdullah, S.R.; Abu Hassan, H.; Kasan, N.A. Response surface methodology (RSM) approach to optimization of coagulation-flocculation of aquaculture wastewater treatment using chitosan from carapace of giant freshwater prawn Macrobrachium rosenbergii. Polymers 2023, 15, 1058. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Amjadi, S.; Mohammadi, M.; Lorenzo, J.M.; Hamishehkar, H. Active gelatin/cress seed gum-based films reinforced with chitosan nanoparticles encapsulating pomegranate peel extract: Preparation and characterization. Food Hydrocolloids 2022, 129, 107620. [Google Scholar] [CrossRef]
- Cao, W.; Yan, J.; Liu, C.; Zhang, J.; Wang, H.; Gao, X.; Yan, H.; Nui, B.; Li, W. Preparation and characterization of catechol-grafted chitosan/gelatin/modified chitosan-AgNP blend films. Carbohydr. Polym. 2020, 247, 116643. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Kurek, M.; Bornaz, S.; Debeaufort, F. Barrier, structural and mechanical properties of bovine gelatin–chitosan blend films related to biopolymer interactions. J. Sci. Food Agric. 2014, 94, 2409–2419. [Google Scholar] [CrossRef]





| Composition | Small Shells (≤5.00 cm in Length) | Big Shells (>5.01 cm in Length) |
|---|---|---|
| Moisture (%) | 0.79 | 0.91 |
| Ash (%) | 42.63 | 92.90 |
| Fat (%) | 0.03 | 0.03 |
| Protein (%) | 5.13 | 4.51 |
| Calcium carbonate (%) | 78.91 | 78.13 |
| Parameters | CHS | CHB | CH |
|---|---|---|---|
| Yield (%) | 0.225 ± 0.022 a | 0.079 ± 0.019 b | - |
| Degree of deacetylation (%, DDA) | 32.71 ± 1.93 c | 52.56 ± 1.27 b | 70.42 ± 1.95 a |
| Solubility (%) | 81.30 ± 2.58 c | 90.00 ± 0.58 b | 100 ± 00 a |
| Water-binding capacity (%, WBC) | 187.97 ± 3.26 b | 209.55 ± 1.53 b | 466.73 ± 3.49 a |
| Fat-binding capacity (%, FBC) | 249.02 ± 2.49 c | 289.23 ± 2.35 b | 452.26 ± 2.99 a |
Color attributes
| 90.51 ± 0.42 a 1.58 ± 0.05 a 5.03 ± 0.12 c | 90.42 ± 0.19 a 1.12 ± 0.05 b 8.48 ± 0.37 b | 86.12 ± 0.07 b 0.13 ± 0.03 c 19.68 ± 0.32 a |
| Properties | CH Film | CH/CHS Film | CH/CHB Film |
|---|---|---|---|
| Thickness (mm) | 0.028 ± 0.0025 b | 0.034 ± 0.0020 a | 0.034 ± 0.0016 a |
| Tensile strength (MPa) | 37.74 ± 0.35 c | 41.81 ± 0.86 b | 45.97 ± 2.34 a |
| Elongation at break (%) | 0.26 ± 0.010 a | 0.25 ± 0.003 a | 0.23 ± 0.004 b |
| Water vapor permeability (×10−7 g m m−2 s−1 Pa−1) | 1.87 ± 0.16 b | 2.30 ± 0.19 a | 1.96 ± 0.20 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewprachu, P.; Jaisan, C. Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study. Polymers 2023, 15, 2816. https://doi.org/10.3390/polym15132816
Kaewprachu P, Jaisan C. Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study. Polymers. 2023; 15(13):2816. https://doi.org/10.3390/polym15132816
Chicago/Turabian StyleKaewprachu, Pimonpan, and Chalalai Jaisan. 2023. "Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study" Polymers 15, no. 13: 2816. https://doi.org/10.3390/polym15132816