The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi
Abstract
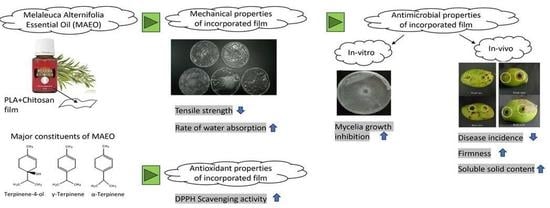
:1. Introduction
2. Materials and Methods
2.1. Extraction of Essential Oil
2.2. Film Preparation
2.3. Mechanical Test of the Film
Tensile Strength
2.4. Antioxidant Activity
2.4.1. Preparation of Film Extract
2.4.2. DPPH Scavenging Activity
2.5. Antifungal Activity
2.6. In Vivo Antifungal Assay on Artificially Wounded and Inoculated Mangoes
2.7. In Vivo Antifungal Assay of Naturally Infected Development on Unwounded Mangoes
2.7.1. Weight Loss
2.7.2. Color Index
2.7.3. Firmness
2.7.4. Soluble Solid Content
2.7.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the Film
3.2. DPPH Antioxidant Test
3.3. In Vitro Antifungal Test
3.4. In Vivo Antifungal Test
3.5. Quality Assessment of Post-Harvest Mango
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dardak, R.A. Trends in Production, Trade, and Consumption of Tropical Fruit in Malaysia. MARDI 2019. [Google Scholar]
- Gomes, A.A.M.; Queiroz, M.V.; Pereira, O.L. Mycofumigation for the Biological Control of Post-Harvest Diseases in Fruits and Vegetables: A Review. Austin J. Biotechnol. Bioeng. 2015, 2, 1051. [Google Scholar]
- Alkan, N.; Fortes, A.M. Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debode, J.; Hemelrijck, W.V.; Creemers, P.; Maes, M. Effect of fungicides on epiphytic yeasts associated with strawberry. Microbiologyopen 2013, 2, 482–491. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Q.; Liang, X.; Fang, S. Fabrication of colloidal stable gliadin-casein nanoparticles for the encapsulation of natamycin: Molecular interactions and antifungal application on cherry tomato. Food Chem. 2022, 391, 133288. [Google Scholar] [CrossRef]
- Hadimani, S.; Supriya, D.; Roopa, K.; Soujanya, S.K.; Rakshata, V.; Netravati, A.; Akshayakumar, V.; Britto, S.D.; Jogaiah, S. Biodegradable hybrid biopolymer film based on carboxy methyl cellulose and selenium nanoparticles with antifungal properties to enhance grapes shelf life. Int. J. Biol. Macromol. 2023, 237, 124076. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based antimicrobial films for food packaging applications. e-Polymers 2008, 93, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Lednev, I.; Salomatina, E.; Ilyina, S.; Zaitsev, S.; Kovylin, R.; Smirnova, L. Development of biodegradable polymer blends based on chitosan and polylactide and study of their properties. Materials 2021, 14, 4900. [Google Scholar] [CrossRef]
- Fathima, P.E.; Panda, S.K.; Ashraf, P.M.; Varghese, T.O.; Bindu, J. Polylactic Acid/Chitosan Films for Packaging of Indian White Prawn (Fenneropenaeus Indicus). Int. J. Biol. Macromol. 2018, 117, 1002–1010. [Google Scholar] [CrossRef]
- Egbo, M.K. A fundamental review on composite materials and some of their applications in biomedical engineering. J. King Saud Univ.-Eng. Sci. 2021, 33, 557–568. [Google Scholar] [CrossRef]
- De Nino, A.; Olivito, F.; Algieri, V.; Costanzo, P.; Jiritano, A.; Tallarida, M.A.; Maiuolo, L. Efficient and Fast Removal of Oils from Water Surfaces via Highly Oleophilic Polyurethane Composites. Toxics 2021, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Wadikar, D.D.; Patki, P.E. Coleus aromaticus: A therapeutic herb with multiple potentials. J. Food Sci. Technol. 2016, 53, 2895–2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Fang, L.P.; Misnan, N.M.; Gopinath, S.C.; Salleh, N.H.M.; Hashim, R.H.R.; Mat, M.H.C. Microwave-assisted solvent-free extraction of essential oil from Coleus aromaticus: Anti-phytopathogenic potential for fruit post-harvesting. 3 Biotech. 2021, 11, 166. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Yang, P.; Li, H.; Liu, Q.; Dong, H.; Duan, Y.; Zhang, J. Plasticization of Poly (lactic) acid Film as a Potential Coating Material. IOP Conf. Ser. Earth Environ. Sci. 2008, 108, 022062. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of active packaging films made from poly(lactic acid)/poly(trimethylene carbonate) incorporated with oregano essential oil. Molecules 2006, 21, 695. [Google Scholar] [CrossRef] [Green Version]
- American Society for Testing and Materials. ASTM, Designación D 882: Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM Inter. 2002. [Google Scholar]
- Muñoz, E.; García-Manrique, J.A. Water absorption behaviour and its effect on the mechanical properties of flax fiber reinforced bioepoxy composites. Int. J. Polym. Sci. 2015, 6, 390275. [Google Scholar]
- Miranda, S.P.; Garnica, O.; Lara-Sagahon, V.A.; Cárdenas, G. Water vapor permeability and mechanical properties of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 173–178. [Google Scholar]
- Mchugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic Edible Films: Modified Procedure for Water Vapor Permeability and Explanation of Thickness Effects. J. Food Sci. 2006, 58, 899–903. [Google Scholar] [CrossRef]
- Yuniarto, K.; Purwanto, S.; Welt, B.A.; Lastriyanto, A. Ascorbic Acid Degradation in Cut Lemon Packaged Using Oxygen Scavenging Active Film During Storage. Makara J. Technol. 2019, 22, 129. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poojary, M.M.; Vishnumurthy, K.A.; Adhikari, A.V. Extraction, characterization and biological studies of phytochemicals from Mammea suriga. J. Pharm. Anal. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal Activity of Essential Oil of Eucalyptus camaldulensis Dehnh. against Selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar] [CrossRef] [Green Version]
- Karim, H.; Boubaker, H.; Askarne, L.; Cherifi, K.; Lakhtar, H.; Msanda, F.; Boudyach, E.H.; Aoumar, A.A.B. Use of Cistus aqueous extracts as botanical fungicides in the control of Citrus sour rot. Microb. Pathog. 2017, 104, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic starch/clay nanocomposites loaded with essential oil constituents as packaging for strawberries−in vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Haggag, W.M. Use of Some Plant Essential Oils as Post-harvest Botanical Fungicides in the Management of Anthracnose Disease of Mango Fruits (Mangi feraindica L.) Caused by Colletotrichum gloeosporioides (Penz). Int. J. Agr. Forest 2013, 3, 1–6. [Google Scholar]
- Gaston, K.K.; Nazaire, K.K.I.I.; Martial, K.F.; Antoine, B.B.B.; Seydou, T.; Coffi, K.; Daouda, K. Antifungal Activity of Essential Oils Extracted from Monodora myristica (Gaertn), Ocimum gratissimum L. and Zingiber officinalis Roscoe on Post-harvest Anthracnose of Mango Fruit (Mangifera indica L.) Variety Kent in Côte d’Ivoire. Int. J. Sci. 2016, 1, 8–18. [Google Scholar]
- Manera, F.J.; Legua, P.; Melgarejo, P.; Brotons, J.M.; Hernández, F.; Martínez, J.J. Determination of a colour index for fruit of pomegranate varietal group “Mollar de Elche”. Sci. Hortic. 2013, 150, 360–364. [Google Scholar] [CrossRef]
- Vilanova, L.; Viñas, I.; Torres, R.; Usall, J.; Buron-Moles, G.; Teixidó, N. Acidification of apple and orange hosts by Penicillium digitatum and Penicillium expansum. Int. J. Food Microbiol. 2014, 178, 39–49. [Google Scholar] [CrossRef]
- Souza, A.C.; Goto, G.E.O.; Mainardi, J.A.; Coelho, A.C.V.; Tadini, C.C. Cassava starch composite films incorporated with cinnamon essential oil: Antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci. Technol. 2013, 54, 346–352. [Google Scholar] [CrossRef]
- Elsabeem, M.Z.; Abdou, E.S. Chitosan based edible films and coatings. A review. Mater. Sci. Eng. C. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Peng, X.; Liang, X.; Fang, S.; Xie, H.; Chen, J.; Meng, Y. Development of antifungal gelatin-based nanocomposite films functionalized with natamycin-loaded zein/casein nanoparticles. Food Hydrocoll. 2021, 113, 106506. [Google Scholar] [CrossRef]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly (lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Nanthakumar, K.; Yeng, C.M.; Chun, K.S. Tensile and water absorption properties of solvent cast biofilms of sugarcane leaves fibre-filled poly(lactic) acid. J. Thermoplast. Compos. Mat. 2020, 33, 289–304. [Google Scholar] [CrossRef]
- Reyes-Chaparro, P.; Gutierrez-Mendez, N.; Salas-Muñoz, E.; Ayala-Soto, J.G.; Chavez-Flores, D.; Hernández-Ochoa, L. Effect of the Addition of Essential Oils and Functional Extracts of Clove on Physicochemical Properties of Chitosan-Based Films. Int. J. Polym. Sci. 2015, 2015, 714254. [Google Scholar] [CrossRef] [Green Version]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Viuda-Martos, M. Evaluation of the antibacterial and antioxidant activities of chitosan edible films incorporated with organic essential oils obtained from four Thymus species. J. Food Sci. Technol. 2016, 53, 3374–3379. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of Antioxidant Activity of Australian Tea Tree (Melaleuca alternifolia) Oil and Its Components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef]
- Ramage, G.; Milligan, S.; Lappin, D.F.; Sherry, L.; Sweeney, P.; Williams, C.; Bagg, J.; Culshaw, S. Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: Potential role in management of oral candidosis in cancer patients. Front. Microbiol. 2012, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.G.d.F.; Hilário, F.; Vilegas, W.; Dos Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.; Bauab, T.M. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int. J. Mol. Sci. 2012, 13, 9260–9277. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, L.; Pastor, C.; Vargas, M.; Chiralt, A.; Gonzalez-Martinez, C.; Chafer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Cháfer, M.; Sánchez-González, L.; González-Martínez, C.; Chiralt, A. Fungal Decay and Shelf Life of Oranges Coated with Chitosan and Bergamot, Thyme, and Tea Tree Essential Oils. J. Food Sci. 2012, 77, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Zaidi, S.; Arshad, M. Postharvest quality assessment of apple during storage at ambient temperature. Heliyon 2021, 7, e07714. [Google Scholar] [CrossRef]
- Harry, T.; Morrison, D. Examining the applicability of Beeswax and Cassave Starch to extend the postharvest life of mangoes (Mangifera indica). Farm Buss. 2018, 10, 39–53. [Google Scholar]
- Abbasi, N.A.; Iqbal, Z.; Maqbool, M.; Hafiz, I.A. Postharvest quality of mango (Mangifera indica L.) fruit as affected by chitosan coating. Pakistan J. Bot. 2009, 41, 343–357. [Google Scholar]
- Rodríguez, F.R.; Cortés, C.G.; Moreno, C.D. Influence of chitosan coatings with citric essential oil on the shelf-life of minimally processed mango (Mangifera indica L. ) Rev. Fac. Nac. Agron. Medellín. 2015, 68, 7679–7688. [Google Scholar] [CrossRef]






| Treatments | Mycelia Growth (mm) | Mycelia Growth Inhibition (%) |
|---|---|---|
| Deionized water as control | - | - |
| Fungicide (Globus 5.5) | 21.7 ± 1.53 | 72.3 ± 2.01 |
| Normal film | 6.0 | - |
| Film + 1.2 wt% essential oil | 11.0 ± 0.50 | 45.5 ± 2.49 |
| Treatment | Colour Index | SSC (%) | Percentage of Weight Loss (%) | Firmness (g/f) |
|---|---|---|---|---|
| Control (distilled water) | −3.38 ± 1.17 | 11.93 ± 0.59 | 13.83 ± 0.31 | 655.0 ± 7.76 |
| Chemical fungicide | −2.62 ± 1.17 | 12.20 ± 0.96 | 10.59 ± 0.09 | 792.77 ± 36.79 |
| The film with 1.2wt% essential oil | −4.14 ± 1.09 | 13.93 ± 0.50 | 9.55 ± 0.08 | 867.83 ± 23.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunny, A.A.N.; Leem, S.J.; Makhtar, M.M.Z.; Zainuddin, N.; Mohd Roslim, M.H.; Raja Hashim, R.H.; Pusphanathan, K.; Siddiqui, M.R.; Alam, M.; Rafatullah, M. The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi. Polymers 2023, 15, 2722. https://doi.org/10.3390/polym15122722
Gunny AAN, Leem SJ, Makhtar MMZ, Zainuddin N, Mohd Roslim MH, Raja Hashim RH, Pusphanathan K, Siddiqui MR, Alam M, Rafatullah M. The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi. Polymers. 2023; 15(12):2722. https://doi.org/10.3390/polym15122722
Chicago/Turabian StyleGunny, Ahmad Anas Nagoor, Siew Juan Leem, Muaz Mohd Zaini Makhtar, Nor’Izzah Zainuddin, Muhammad Huzaifah Mohd Roslim, Raja Hasnida Raja Hashim, Kavita Pusphanathan, Masoom Raza Siddiqui, Mahboob Alam, and Mohd Rafatullah. 2023. "The Use of Essential Oil Embedded in Polylactic Acid/Chitosan-Based Film for Mango Post-Harvest Application against Pathogenic Fungi" Polymers 15, no. 12: 2722. https://doi.org/10.3390/polym15122722