Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug Encapsulation
2.3. Particle Size of Pure and Drug-Loaded Micellar Systems
2.4. In Vitro Drug Release
2.5. Cell Viability Assays
3. Results
3.1. Drug-Loading Capacity of Polymeric Micelles
3.2. Micellar Size of Drug-Loaded Polymeric System
3.3. In Vitro Release Profile from Polymeric Micelles
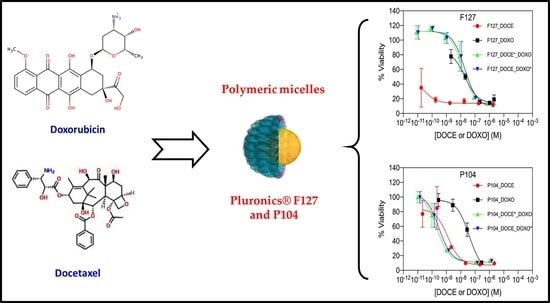
3.4. Cell Viability Assays of Individual and Combined Micelles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L. Drug Targeting Strategies in Cancer Treatment: An Overview. Mini-Rev. Med. Chem. 2011, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Gaur, U.; Ghosh, P.C.; Maitra, A.N. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J. Control. Release 2001, 74, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Lee, H.; Kim, H.; Kong, W.; Fim, Y.; Cui, Z.; Park, K.; Kim, S.; Lee, G.; Seo, S. Bio-distribution and anti-tumor efficacy of PEG/PLA nano particles loaded doxorubicin. J. Drug Target. 2007, 15, 279–284. [Google Scholar] [CrossRef]
- Battaglia, G.; Ryan, A.J. Bilayers and interdigitation in block copolymer vesicles. J. Am. Chem. Soc. 2005, 127, 8757–8764. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Villar-Alvarez, E.; Figueroa-Ochoa, E.; Barbosa, S.; Soltero, J.F.A.; Taboada, P.; Mosquera, V. Reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) block copolymers with lengthy hydrophilic blocks as efficient single and dual drug-loaded nanocarriers with synergistic toxic effects on cancer cells. RSC Adv. 2015, 5, 52105–52120. [Google Scholar] [CrossRef]
- Alexandridis, P.; Nivaggioli, T.; Hatton, T.A. Temperature Effects on Structural Properties of Pluroncc P104 and F108 PEO-PPO-PEO Block Copolymer Solutions. Langmuir 1995, 11, 1468–1476. [Google Scholar] [CrossRef]
- Elsabahy, M.; Perron, M.É.; Bertrand, N.; Yu, G.E.; Leroux, J.C. Solubilization of docetaxel in poly(ethylene oxide)-block-poly(butylene/styrene oxide) micelles. Biomacromolecules 2007, 8, 2250–2257. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Brynskikh, A.; Sharma, A.; Li, Y.; Boska, M.; Gong, N.; Mosley, R.; Alakhov, V.; Gendelman, H.; et al. Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. J. Control. Release 2010, 143, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kabanov, A.V. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2008, 130, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, H.; Liu, X.; Zhang, M.; Zhai, G. Preparation and evaluation in vitro and in vivo of docetaxel loaded mixed micelles for oral administration. Colloids Surf. B Biointerfaces 2014, 114, 20–27. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rivory, L.P. Clinical pharmacokinetics of docetaxel. Clin. Pharmacokinet. 1999, 36, 99–114. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bhatt, A.; Castro, E.; Mishra, A.; Chuttani, K.; Dwarakanath, B.; Schatz, C.; Le, J.; Misra, A.; Lecommandoux, S. In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol. Biosci. 2010, 10, 503–512. [Google Scholar] [CrossRef]
- Moes, J.J.; Koolen, S.L.W.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H.; Nuijen, B. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int. J. Pharm. 2011, 420, 244–250. [Google Scholar] [CrossRef]
- Moro, S.; Beretta, G.L.; Ben, D.D.; Nitiss, J.; Palumbo, M.; Capranico, G. Interaction model for anthracycline activity against DNA topoisomerase II. Biochemistry 2004, 43, 7503–7513. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, Y.S.; Kim, D.K. Doxorubicin exerts cytotoxic effects through cell cycle arrest and fas-mediated cell death. Pharmacology 2009, 84, 300–309. [Google Scholar] [CrossRef]
- Cuong, N.; Jiang, J.; Hsiesh, M.; Rd, V.B.; City, H.C.M. Doxorubicin Delivery by Copolymeric Nanoparticle for Treatment of Breast Cancer. IFMBE Proc. 2010, 27, 159–162. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Cambón, A.; Rey-Rico, A.; Mistry, D.; Brea, J.; Loza, M.I.; Attwood, D.; Barbosa, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; et al. Doxorubicin-loaded micelles of reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) block copolymers as efficient ‘active’ chemotherapeutic agents. Int. J. Pharm. 2013, 445, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Šubr, V. Polymeric anticancer drugs with pH-controlled activation. Adv. Drug Deliv. Rev. 2004, 56, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Chen, H.; Qin, J.; Liang, J.; Kong, D.; Liu, T.; Lv, F. Real-Time Imaging Tracking of a Dual Fluorescent Drug Delivery System Based on Zinc Phthalocyanine-Incorporated Hydrogel. ACS Biomater. Sci. Eng. 2016, 2, 2001–2010. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Research paper enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly(Amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, S.; Yu, J.; Park, T.; Hyeon, T. Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer-targeted imaging and magnetically guided drug delivery. Adv. Mater. 2008, 20, 478–483. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 148124, Docetaxel. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Docetaxel (accessed on 12 April 2023).
- Yalkowsky, S.H.; He, Y. Handbook of Aqueous Solubility Data; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Crothers, M.; Zhou, Z.; Ricardo, N.; Yang, Z.; Taboada, P.; Chaibundit, C.; Attwood, D.; Booth, C. Solubilisation in aqueous micellar solutions of block copoly(oxyalkylene)s. Int. J. Pharm. 2005, 293, 91–100. [Google Scholar] [CrossRef]
- Cambón, A.; Barbosa, S.; Rey-Rico, A.; Figueroa-Ochoa, E.B.; Soltero, J.F.; Yeates, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; Mosquera, V. Poly(ethylene oxide)-poly(styrene oxide)-poly(ethylene oxide) copolymers: Micellization, drug solubilization, and gelling features. J. Colloid Interface Sci. 2012, 387, 275–284. [Google Scholar] [CrossRef]
- Taboada, P.; Velasquez, G.; Barbosa, S.; Castelletto, V.; Nixon, S.; Yang, Z.; Heatley, F.; Hamley, I.; Ashford, M.; Mosquera, V.; et al. Block copolymers of ethylene oxide and phenyl glycidyl ether: Micellization, gelation, and drug solubilization. Langmuir 2005, 21, 5263–5271. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Wang, J.; Chen, T.; Wang, Y.; Tan, Y.; Su, H.; Chan, K.; Chen, H. Probing the kinetics of short-distance drug release from nanocarriers to nanoacceptors. Angew. Chemie-Int. Ed. 2010, 49, 8426–8430. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Pikabea, A.; Villar-Álvarez, E.; Forcada, J.; Taboada, P. pH-controlled doxorubicin delivery from PDEAEMA-based nanogels. J. Mol. Liq. 2018, 266, 321–329. [Google Scholar] [CrossRef]
- Fernández, A.; Santos, G.; Esteves, F. Estudio in vitro de liberación de fármacos desde un biomaterial compuesto. CENIC Ciencias Químicas 2010, 41, 1–8. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Wang, L.; Zhang, J.; Zhang, N. Docetaxel-Loaded pluronic P123 polymeric micelles: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2011, 12, 1684–1696. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Gonzalez-Lopez, J.; Fernandez-Tarrio, M.; Sandez-Macho, I.; Concheiro, A. Tetronic micellization, gelation and drug solubilization: Influence of pH and ionic strength. Eur. J. Pharm. Biopharm. 2007, 66, 244–252. [Google Scholar] [CrossRef]
- Crothers, M.; Attwood, D.; Collett, J.; Yang, Z.; Booth, C.; Taboada, P.; Mosquera, V.; Ricardo, N.; Martini, L. Micellization and gelation of diblock copolymers of ethylene oxide and styrene oxide in aqueous solution. Langmuir 2002, 18, 8685–8691. [Google Scholar] [CrossRef]
- Figueroa-Ochoa, E.B.; Villar-Álvarez, E.; Cambón, A.; Mistry, D.; Llovo, J.; Attwood, D.; Barbosa, S.; Soltero, J.A.; Taboada, P. Lenghty reverse poly(butylene oxide)-poly(ethylene oxide)-poly(butylene oxide) polymeric micelles and gels for sustained release of antifungal drugs. Int. J. Pharm. 2016, 510, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012, 64, 237–245. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Shin, H.C.; Alani, A.W.G.; Rao, D.A.; Rockich, N.C.; Kwon, G.S. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J. Control. Release 2009, 140, 294–300. [Google Scholar] [CrossRef]
- Berko, Y.A.; Funmilola, A.F.; Akala, E.O. Fabrication of Paclitaxel and 17AAG-loaded Poly-ε-Caprolactone Nanoparticles for Breast Cancer Treatment. J. Pharm. Drug Deliv. Res. 2021, 10, 196. [Google Scholar]
- Hasenstein, J.R.; Shin, H.C.; Kasmerchak, K.; Buehler, D.; Kwon, G.S.; Kozak, K.R. Antitumor activity of triolimus: A novel multidrug-loaded micelle containing paclitaxel, rapamycin, and 17-AAG. Mol. Cancer Ther. 2012, 11, 2233–2242. [Google Scholar] [CrossRef]
- Baggi, R.B.; Kilaru, N.B. Calculation of predominant drug release mechanism using Peppas-Sahlin model, Part-I (substitution method): A linear regression approach. Asian J. Pharm. Technol. 2016, 6, 223–230. [Google Scholar] [CrossRef]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Speth, P.A.J.; van Hoesel, Q.G.C.M.; Haanen, C. Clinical Pharmacokinetics of Doxorubicin. Clin. Pharmacokinet. 1988, 15, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, L.J.; Sales, T.; Clarance, P.; Agastian, P.; Kim, Y.; Mahmoud, A.H.; Mohamed, S.; Tack, J.C.; Na, S.W.; Kim, H. In-vitro phytopharmacological and anticancer activity of Loranthus Longiflorus Desv. Var. Falcatuskurz against the human lung cancer cells. J. King Saud Univ.-Sci. 2020, 32, 1246–1253. [Google Scholar] [CrossRef]
- Benyettou, F.; Fahs, H.; Elkharrag, R.; Bilbeisi, R.; Asma, B.; Rezgui, R.; Motte, L.; Magzoub, M.; Brandel, J.; Olsen, J.; et al. Selective growth inhibition of cancer cells with doxorubicin-loaded CB[7]-modified iron-oxide nanoparticles. RSC Adv. 2017, 7, 23827–23834. [Google Scholar] [CrossRef]
- Cambón, A.; Brea, J.; Loza, M.; Alvarez-Lorenzo, C.; Concheiro, A.; Barbosa, S.; Taboada, P.; Mosquera, V. Cytocompatibility and P-glycoprotein inhibition of block copolymers: Structure-activity relationship. Mol. Pharm. 2013, 10, 3232–3241. [Google Scholar] [CrossRef]
- Altamimi, A.S.; El-Azab, A.S.; Abdelhamid, S.; Alamri, M.; Bayoumi, A.; Alqahtani, S.; Alabbas, A.; Altharawi, A.; Alossaimi, M.; Mohamed, M. Synthesis, Anticancer Screening of Some Novel Trimethoxy Quinazolines and VEGFR2, EGFR Tyrosine Kinase Inhibitors Assay; Molecular Docking Studies. Molecules 2021, 26, 2992. [Google Scholar] [CrossRef]
- Afzal, S.M.; Naidu, V.G.M.; Harishankar, N.; Kishan, V. Albumin anchored docetaxel lipid nanoemulsion for improved targeting efficiency—Preparation, characterization, cytotoxic, antitumor and in vivo imaging studies. Drug Deliv. 2016, 23, 1355–1363. [Google Scholar] [CrossRef]
- Balcer-Kubiczek, E.K.; Attarpour, M.; Jiang, J.; Kennedy, A.S.; Suntharalingam, M. Cytotoxicity of docetaxel (Taxotere®) used as a single agent and in combination with radiation in human gastric, cervical and pancreatic cancer cells. Chemotherapy 2006, 52, 231–240. [Google Scholar] [CrossRef]
- Tsakalozou, E.; Eckman, A.M.; Bae, Y. Combination effects of docetaxel and doxorubicin in hormone-refractory prostate cancer cells. Biochem. Res. Int. 2012, 1, 832059. [Google Scholar] [CrossRef]


 ) F127 and (
) F127 and ( ) P104 polymer micelles.
) P104 polymer micelles.
 ) F127 and (
) F127 and ( ) P104 polymer micelles.
) P104 polymer micelles.



| * D/C (w/w%) | (μg) | D.L. (wt%) | E.E. (wt%) | Scp (mg·g−1) | (μg) | D.L. (wt%) | E.E. (wt%) | Scp (mg·g−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| DOCE loading (PEO100PPO65PEO100) | DOXO loading (PEO100PPO65PEO100) | |||||||||
| 0.05 | 6.45 | ±0.93 | 0.031 | 64.5 | 0.313 | 6.02 | ±0.91 | 0.030 | 60.2 | 0.303 |
| 0.10 | 11.82 | ±0.99 | 0.058 | 59.1 | 0.583 | 10.42 | ±1.43 | 0.049 | 52.1 | 0.495 |
| 0.15 | 16.97 | ±0.75 | 0.088 | 56.6 | 0.876 | 14.34 | ±1.98 | 0.068 | 47.8 | 0.685 |
| 0.20 | 21.76 | ±0.54 | 0.115 | 54.4 | 1.147 | 17.31 | ±1.90 | 0.085 | 43.3 | 0.849 |
| 0.25 | 28.42 | ±0.90 | 0.142 | 56.8 | 1.424 | 22.90 | ±1.74 | 0.114 | 45.8 | 1.145 |
| 0.30 | 27.61 | ±0.81 | 0.141 | 46.0 | 1.408 | 24.50 | ±1.29 | 0.117 | 40.8 | 1.171 |
| DOCE loading (PEO27PPO61PEO27) | DOXO loading (PEO27PPO61PEO27) | |||||||||
| 0.02 | 8.50 | ±0.02 | 0.017 | 85.0 | 0.170 | 8.75 | ±0.49 | 0.017 | 87.5 | 0.175 |
| 0.04 | 15.77 | ±0.40 | 0.032 | 78.9 | 0.315 | 15.22 | ±0.74 | 0.030 | 76.1 | 0.304 |
| 0.06 | 21.61 | ±1.42 | 0.043 | 72.0 | 0.432 | 21.75 | ±0.63 | 0.043 | 72.5 | 0.435 |
| 0.08 | 35.40 | ±1.24 | 0.071 | 88.5 | 0.710 | 30.41 | ±1.53 | 0.061 | 76.0 | 0.609 |
| 0.10 | 40.41 | ±0.57 | 0.081 | 80.8 | 0.808 | 31.39 | ±1.14 | 0.063 | 62.8 | 0.628 |
| 0.12 | 42.15 | ±0.63 | 0.084 | 70.3 | 0.843 | 32.55 | ±1.48 | 0.065 | 54.2 | 0.651 |
| Copolymers | [DOXO/DOCE] (w/w%) | DOXO (μg) | DOCE (μg) | D.L. (wt%) | E.E. (wt%) | Scp (mg·g−1) | ||
|---|---|---|---|---|---|---|---|---|
| PEO100PPO65PEO100 (2 wt%) | 100/0 | 22.90 | ±1.74 | 0.00 | 0.00 | 0.046 | 45.8 | 1.141 |
| 75/25 | 16.77 | ±2.08 | 6.69 | ±0.38 | 0.047 | 46.9 | 1.173 | |
| 50/50 | 11.76 | ±0.91 | 12.86 | ±1.09 | 0.049 | 49.2 | 1.231 | |
| 25/75 | 4.89 | ±0.1 | 19.35 | ±1.43 | 0.049 | 48.5 | 1.214 | |
| 0/100 | 0.00 | 0.00 | 28.42 | ±0.90 | 0.057 | 56.8 | 1.424 | |
| PEO27PPO61PEO27 (5 wt%) | 100/0 | 30.41 | ±1.53 | 0.00 | 0.00 | 0.076 | 76.0 | 0.609 |
| 75/25 | 22.33 | ±0.67 | 8.36 | ±0.47 | 0.077 | 76.7 | 0.614 | |
| 50/50 | 15.34 | ±0.71 | 16.08 | ±1.37 | 0.079 | 78.6 | 0.628 | |
| 25/75 | 6.55 | ±0.49 | 24.22 | ±1.79 | 0.077 | 76.9 | 0.615 | |
| 0/100 | 0.00 | 0.00 | 35.40 | ±1.24 | 0.089 | 88.5 | 0.712 | |
| Diffusion Models | F127_DOCE | P104_DOCE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | ||||||
| Higuchi | k | 14.05 | ±0.92 | 18.96 | ±1.25 | 10.90 | ±0.77 | 15.36 | ±2.32 |
| R2 | 0.973 | 0.987 | 0.985 | 0.985 | |||||
| MSE | 26.87 | 25.45 | 8.43 | 17.53 | |||||
| MSC | 3.59 | 4.17 | 4.12 | 4.08 | |||||
| Korsmeyer–Peppas | k1 | 17.33 | ±3.61 | 21.11 | ±2.87 | 13.53 | ±1.84 | 18.41 | ±3.99 |
| n | 0.44 | ±0.04 | 0.47 | ±0.02 | 0.44 | ±0.02 | 0.45 | ±0.04 | |
| R2 | 0.985 | 0.990 | 0.993 | 0.992 | |||||
| MSE | 15.89 | 21.33 | 3.67 | 9.61 | |||||
| MSC | 3.90 | 4.24 | 4.83 | 4.60 | |||||
| Peppas–Sahlin | k2 | 15.60 | ±3.37 | 18.88 | ±1.62 | 12.82 | ±1.86 | 17.90 | ±3.72 |
| k3 | −0.68 | ±0.31 | −0.65 | ±0.17 | 1.08 | ±0.12 | 0.79 | ±1.01 | |
| m | 0.59 | ±0.03 | 0.60 | ±0.04 | 0.37 | ±0.01 | 0.41 | ±0.04 | |
| R2 | 0.993 | 0.995 | 0.994 | 0.993 | |||||
| MSE | 7.72 | 10.11 | 3.47 | 9.23 | |||||
| MSC | 4.57 | 4.92 | 4.86 | 4.60 | |||||
| Diffusion Models | F127_DOXO | P104_DOXO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | ||||||
| Higuchi | k | 9.74 | ±0.04 | 15.85 | ±0.52 | 11.28 | ±0.77 | 16.02 | ±2.51 |
| R2 | 0.876 | 0.979 | 0.981 | 0.984 | |||||
| MSE | 48.95 | 25.96 | 10.81 | 22.07 | |||||
| MSC | 1.84 | 3.75 | 3.14 | 4.06 | |||||
| Korsmeyer–Peppas | k1 | 16.20 | ±1.21 | 19.89 | ±2.39 | 16.07 | ±5.01 | 17.89 | ±5.10 |
| n | 0.34 | ±0.02 | 0.43 | ±0.03 | 0.40 | ±0.08 | 0.47 | ±0.06 | |
| R2 | 0.954 | 0.990 | 0.987 | 0.990 | |||||
| MSE | 19.36 | 12.86 | 7.75 | 14.57 | |||||
| MSC | 2.72 | 4.32 | 3.98 | 4.37 | |||||
| Peppas–Sahlin | k2 | 14.84 | ±1.76 | 19.17 | ±2.21 | 16.28 | ±5.51 | 17.06 | ±5.74 |
| k3 | −0.99 | ±0.26 | −0.73 | ±0.26 | −0.15 | ±0.72 | 1.38 | ±1.20 | |
| m | 0.54 | ±0.05 | 0.52 | ±0.01 | 0.40 | ±0.05 | 0.40 | ±0.01 | |
| R2 | 0.981 | 0.992 | 0.988 | 0.991 | |||||
| MSE | 8.30 | 10.42 | 7.59 | 12.90 | |||||
| MSC | 3.47 | 4.47 | 4.08 | 4.45 | |||||
| Diffusion Models | F127_DOCE-DOXO | P104_DOCE-DOXO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | ||||||
| Higuchi | k | 9.75 | ±0.42 | 15.63 | ±0.65 | 10.18 | ±0.24 | 16.81 | ±0.55 |
| R2 | 0.985 | 0.990 | 0.985 | 0.986 | |||||
| MSE | 7.02 | 11.75 | 7.16 | 17.23 | |||||
| MSC | 3.99 | 4.47 | 4.06 | 4.19 | |||||
| Korsmeyer–Peppas | k1 | 11.06 | ±0.92 | 17.91 | ±0.38 | 12.24 | ±0.93 | 19.18 | ±1.86 |
| n | 0.46 | ±0.02 | 0.46 | ±0.01 | 0.44 | ±0.02 | 0.45 | ±0.02 | |
| R2 | 0.989 | 0.994 | 0.991 | 0.991 | |||||
| MSE | 5.73 | 8.13 | 4.38 | 11.63 | |||||
| MSC | 4.09 | 4.78 | 4.53 | 4.50 | |||||
| Peppas–Sahlin | k2 | 11.06 | ±0.92 | 17.55 | ±0.55 | 11.59 | ±1.36 | 17.47 | ±1.13 |
| k3 | −0.22 | ±0.09 | −0.23 | ±0.37 | 0.97 | ±0.54 | 2.36 | ±0.69 | |
| m | 0.50 | ±0.01 | 0.49 | ±0.06 | 0.38 | ±0.02 | 0.37 | ±0.02 | |
| R2 | 0.989 | 0.994 | 0.992 | 0.993 | |||||
| MSE | 5.57 | 7.87 | 4.05 | 10.20 | |||||
| MSC | 4.16 | 4.83 | 4.56 | 4.60 | |||||
| Micellar System | IC50 (µM) | R2 |
|---|---|---|
| P104 empty | 82.32 | 0.943 |
| P104_DOCE | 0.00126 | 0.888 |
| P104_DOXO | 0.03270 | 0.975 |
| P104_DOCE-DOXO | 0.0002 a 0.0003 b | 0.966 a 0.966 b |
| F127 empty | >1 × 1011 | - |
| F127_DOCE | 0.00003 | 0.487 |
| F127_DOXO | 0.02030 | 0.956 |
| F127_DOCE-DOXO | 0.0144 a 0.0195 b | 0.959 a 0.959 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Saucedo, R.A.; Gómez-López, J.C.; Villanueva-Briseño, A.A.; Topete, A.; Soltero-Martínez, J.F.A.; Mendizábal, E.; Jasso-Gastinel, C.F.; Taboada, P.; Figueroa-Ochoa, E.B. Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs. Polymers 2023, 15, 2249. https://doi.org/10.3390/polym15102249
Gutiérrez-Saucedo RA, Gómez-López JC, Villanueva-Briseño AA, Topete A, Soltero-Martínez JFA, Mendizábal E, Jasso-Gastinel CF, Taboada P, Figueroa-Ochoa EB. Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs. Polymers. 2023; 15(10):2249. https://doi.org/10.3390/polym15102249
Chicago/Turabian StyleGutiérrez-Saucedo, Ramón A., Julio C. Gómez-López, Adrián A. Villanueva-Briseño, Antonio Topete, J. F. Armando Soltero-Martínez, Eduardo Mendizábal, Carlos F. Jasso-Gastinel, Pablo Taboada, and Edgar B. Figueroa-Ochoa. 2023. "Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs" Polymers 15, no. 10: 2249. https://doi.org/10.3390/polym15102249