Structural Behavior of Amphiphilic Triblock Copolymer P104/Water System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Triblock Copolymer P104/H2O Solutions Preparation
2.2. Crossed-Light Polarized Microscopy
2.3. Viscosity Measurements
2.4. Density and Ultrasound Velocity Measurements
2.5. Dynamic Light Scattering Measurements
2.6. Turbidity Measurements
2.7. Rheometry
- Strain sweeps: in order to obtain the linear viscoelastic regimes, the oscillation strain sweeps were performed at a controlled angular frequency of 10 rad/s in a strain range between 0.1% and 100%, using 10 points per decade. A strain sweep was performed for each P104 sample at a selected temperature depending on the chosen concentration.
- Temperature sweeps: these sweeps were performed for each P104 sample using a strain value in the linear viscoelastic region, selected from previous experiments, applying an angular frequency of 10 rad/s in a temperature range from 1 to 90 °C, with a heating rate of 1 °C/min.
3. Results and Discussion
3.1. Visual Observations
3.2. P104 Micellization in Water Evaluated through Viscosity Measurements
3.3. P104 Micellization Process Studied through Density and Sound Velocity Measurements
3.4. Evaluation of the Hydration Number of P104/Water System
3.5. Morphology of P104 Micelles in Water by Dynamic Light Scattering (DLS)
3.6. Turbidity Measurements
3.7. Rheological Measurements
3.7.1. Linear Viscoelastic Region
3.7.2. Temperature Sweeps
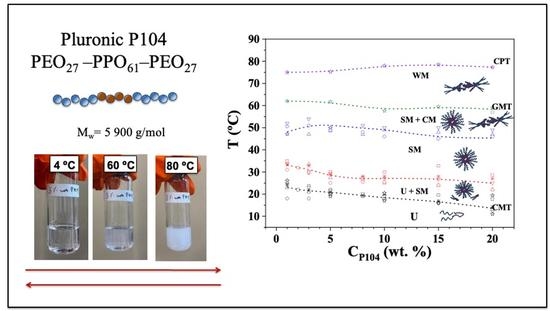
3.8. Temperature-Composition Phase Diagram of P104/Water in the Dilute and Semi-Dilute Regimes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Alexandridis, P.; Alan Hatton, T. Poly(Ethylene Oxide)-poly(Propylene Oxide)-poly(Ethylene Oxide) Block Copolymer Surfactants in Aqueous Solutions and at Interfaces: Thermodynamics, Structure, Dynamics, and Modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Karayianni, M.; Pispas, S. Self-Assembly of Amphiphilic Block Copolymers in Selective Solvents. In Fluorescence Studies of Polymer Containing Systems; Procházka, K., Ed.; Springer Series on Fluorescence; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–63. [Google Scholar] [CrossRef]
- Alexandridis, P. Thermodynamics and Dynamics of Micellization and Micelle-Solute Interactions in Block-Copolymer and Reverse Micellar Systems. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1994. [Google Scholar]
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Ma, J.W.; Li, X.; Tang, P.; Yang, Y. Self-Assembly of Amphiphilic ABC Star Triblock Copolymers and Their Blends with AB Diblock Copolymers in Solution: Self-Consistent Field Theory Simulations. J. Phys. Chem. B 2007, 111, 1552–1558. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic Block Copolymers for Drug Delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar Formulations for Drug Delivery Based on Mixtures of Hydrophobic and Hydrophilic Pluronic® Block Copolymers. J. Control Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® Block Copolymers as Novel Polymer Therapeutics for Drug and Gene Delivery. J. Control Release 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Bravo-Anaya, L.M.; Landazuri Gómez, G.; Figueroa-Ochoa, E.; Carvajal Ramos, F.; Martínez, J.F.A.S.; Rharbi, Y. Exchange Dynamics between Amphiphilic Block Copolymers and Lipidic Membranes through Hydrophobic Pyrene Probe Transfer. RSC Adv. 2018, 8, 39444–39454. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric Micelles: Authoritative Aspects for Drug Delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Schacher, F.; Walther, A.; Müller, A.H.E. Dynamic Multicompartment-Core Micelles in Aqueous Media. Langmuir 2009, 25, 10962–10969. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Lavasanifar, A. Amphiphilic Block Copolymer Based Nanocarriers for Drug and Gene Delivery. In Intracellular Delivery: Fundamentals and Applications; Prokop, A., Ed.; Fundamental Biomedical Technologies; Springer: Dordrecht, The Netherlands, 2011; pp. 251–289. [Google Scholar] [CrossRef]
- Kim, T.; Seo, H.J.; Choi, J.S.; Jang, H.-S.; Baek, J.; Kim, K.; Park, J.-S. PAMAM-PEG-PAMAM: Novel Triblock Copolymer as a Biocompatible and Efficient Gene Delivery Carrier. Biomacromolecules 2004, 5, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Yasen, W.; Dong, R.; Zhou, L.; Wu, J.; Cao, C.; Aini, A.; Zhu, X. Synthesis of a Cationic Supramolecular Block Copolymer with Covalent and Noncovalent Polymer Blocks for Gene Delivery. ACS Appl. Mater. Interfaces 2017, 9, 9006–9014. [Google Scholar] [CrossRef] [PubMed]
- Yasen, W.; Dong, R.; Zhou, L.; Huang, Y.; Guo, D.; Chen, D.; Li, C.; Aini, A.; Zhu, X. Supramolecular Block Copolymers for Gene Delivery: Enhancement of Transfection Efficiency by Charge Regulation. Chem. Commun. 2017, 53, 12782–12785. [Google Scholar] [CrossRef] [PubMed]
- Alakhov, V.; Klinski, E.; Li, S.; Pietrzynski, G.; Venne, A.; Batrakova, E.; Bronitch, T.; Kabanov, A. Block Copolymer-Based Formulation of Doxorubicin. From Cell Screen to Clinical Trials. Colloids Surf. B Biointerfaces 1999, 16, 113–134. [Google Scholar] [CrossRef]
- Alakhova, D.Y.; Kabanov, A.V. Pluronics and MDR Reversal: An Update. Mol. Pharm. 2014, 11, 2566–2578. [Google Scholar] [CrossRef]
- Waton, G.; Michels, B.; Zana, R. Dynamics of Block Copolymer Micelles in Aqueous Solution. Macromolecules 2001, 34, 907–910. [Google Scholar] [CrossRef]
- Michels, B.; Waton, G.; Zana, R. Evidence of Micelle Growth in Aqueous Solutions of the Amphiphilic Poly(Ethylene Oxide)–Poly(Propylene Oxide)–Poly(Ethylene Oxide) Triblock Copolymers from Differential Scanning Microcalorimetry. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183, 55–65. [Google Scholar] [CrossRef]
- Wanka, G.; Hoffmann, H.; Ulbricht, W. The Aggregation Behavior of Poly-(Oxyethylene)-Poly-(Oxypropylene)-Poly-(Oxyethylene)-Block-Copolymers in Aqueous Solution. Colloid Polym. Sci. 1990, 268, 101–117. [Google Scholar] [CrossRef]
- Mortensen, K.; Brown, W.Y.N. Poly (Ethylene Oxide)-Poly (Propylene Oxide)-Poly (Ethylene Oxide) Triblock Copolymers in Aqueous Solution. The Influence of Relative Block Size. Macromolecules 1993, 26, 4128–4135. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Svaneborg, C. Scattering from Block Copolymer Micelles. Curr. Opin. Colloid Interface Sci. 2002, 7, 158–166. [Google Scholar] [CrossRef]
- Beaton, L.; Zhang, S.; Kruk, M. Formation of Double-Helical Structures by Silica Nanotubes Templated by Mixtures of Common Nonionic Surfactants in Aqueous Solutions. ACS Nano 2021, 15, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Awoke, Y.; Chebude, Y.; Díaz, I. Controlling Particle Morphology and Pore Size in the Synthesis of Ordered Mesoporous Materials. Molecules 2020, 25, 4909. [Google Scholar] [CrossRef] [PubMed]
- Hvidt, S.; Keiding, K. Rheology and Structures of EO-PO-EO Block Copolymers in Aqueous Solutions. Ann. Trans. Nordic Rheol. Soc. 2009, 17, 103–107. [Google Scholar]
- Flodström, K.; Alfredsson, V. Influence of the Block Length of Triblock Copolymers on the Formation of Mesoporous Silica. Microporous Mesoporous Mater. 2003, 59, 167–176. [Google Scholar] [CrossRef]
- Kipkemboi, P.; Fogden, A.; Alfredsson, V.; Flodström, K. Triblock Copolymers as Templates in Mesoporous Silica Formation: Structural Dependence on Polymer Chain Length and Synthesis Temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Single-Step Synthesis and Stabilization of Metal Nanoparticles in Aqueous Pluronic Block Copolymer Solutions at Ambient Temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef]
- Linton, P.; Wennerström, H.; Alfredsson, V. Controlling Particle Morphology and Size in the Synthesis of Mesoporous SBA-15 Materials. Phys. Chem. Chem. Phys. 2010, 12, 3852–3858. [Google Scholar] [CrossRef]
- Garripelli, V.K.; Kim, J.-K.; Namgung, R.; Kim, W.J.; Repka, M.A.; Jo, S. A Novel Thermosensitive Polymer with pH-Dependent Degradation for Drug Delivery. Acta Biomater. 2010, 6, 477–485. [Google Scholar] [CrossRef]
- Garripelli, V.K.; Kim, J.-K.; Son, S.; Kim, W.J.; Repka, M.A.; Jo, S. Matrix Metalloproteinase-Sensitive Thermogelling Polymer for Bioresponsive Local Drug Delivery. Acta Biomater. 2011, 7, 1984–1992. [Google Scholar] [CrossRef]
- Whang, C.-H.; Lee, H.K.; Kundu, S.; Murthy, S.N.; Jo, S. Pluronic-Based Dual-Stimuli Sensitive Polymers Capable of Thermal Gelation and pH-Dependent Degradation for in Situ Biomedical Application. J. Appl. Polym. Sci. 2018, 135, 46552. [Google Scholar] [CrossRef]
- Ganguly, R.; Kuperkar, K.; Parekh, P.; Aswal, V.K.; Bahadur, P. Phenol Solubilization in Aqueous Pluronic® Solutions: Investigating the Micellar Growth and Interaction as a Function of Pluronic® Composition. J. Colloid Interface Sci. 2012, 378, 118–124. [Google Scholar] [CrossRef]
- Causse, J.; Lagerge, S.; de Menorval, L.C.; Faure, S. Micellar Solubilization of Tributylphosphate in Aqueous Solutions of Pluronic Block Copolymers: Part I. Effect of the Copolymer Structure and Temperature on the Phase Behavior. J. Colloid Interface Sci. 2006, 300, 713–723. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, K.; Bahadur, A.; Marangoni, G.; Bahadur, P. Interaction and Solubilization of Some Phenolic Antioxidants in Pluronic® Micelles. Colloids Surf. B 2011, 86, 319–326. [Google Scholar] [CrossRef]
- Khimani, M.; Ganguly, R.; Aswal, V.K.; Nath, S.; Bahadur, P. Solubilization of Parabens in Aqueous Pluronic Solutions: Investigating the Micellar Growth and Interaction as a Function of Paraben Composition. J. Phys. Chem. B 2012, 116, 14943–14950. [Google Scholar] [CrossRef] [PubMed]
- Linley, S.; Thomson, N.R.; McVey, K.; Sra, K.; Gu, F.X. Influence of Pluronic Coating Formulation on Iron Oxide Nanoparticle Transport in Natural and Oil-Impacted Sandy Aquifer Media. Can. J. Chem. Eng. 2020, 98, 642–649. [Google Scholar] [CrossRef]
- Brüsewitz, C.; Schendler, A.; Funke, A.; Wagner, T.; Lipp, R. Novel Poloxamer-Based Nanoemulsions to Enhance the Intestinal Absorption of Active Compounds. Int. J. Pharm. 2007, 329, 173–181. [Google Scholar] [CrossRef]
- Kaizu, K.; Alexandridis, P. Effect of Surfactant Phase Behavior on Emulsification. J. Colloid Interface Sci. 2016, 466, 138–149. [Google Scholar] [CrossRef]
- Varshney, M.; Morey, T.E.; Shah, D.O.; Flint, J.A.; Moudgil, B.M.; Seubert, C.N.; Dennis, D.M. Pluronic Microemulsions as Nanoreservoirs for Extraction of Bupivacaine from Normal Saline. J. Am. Chem. Soc. 2004, 126, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wu, D. Microencapsulation of N-Dodecane into Zirconia Shell Doped with Rare Earth: Design and Synthesis of Bifunctional Microcapsules for Photoluminescence Enhancement and Thermal Energy Storage. Energy 2016, 97, 113–126. [Google Scholar] [CrossRef]
- Heerema, L.; Cakali, D.; Roelands, M.; Goetheer, E.; Verdoes, D.; Keurentjes, J. Micellar Solutions of PEO–PPO–PEO Block Copolymers for in Situ Phenol Removal from Fermentation Broth. Sep. Purif. Technol. 2010, 73, 319–326. [Google Scholar] [CrossRef]
- Mandal, M.; Farid, G.; Kruk, M. Swollen Mixed Pluronic Surfactant Micelles as Templates for Mesoporous Nanotubes with Diverse Bridged-Organosilica Frameworks. J. Colloid Interface Sci. 2018, 524, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Farid, G.; Kruk, M. Silica Nanotubes with Widely Adjustable Inner Diameter and Ordered Silicas with Ultralarge Cylindrical Mesopores Templated by Swollen Micelles of Mixed Pluronic Triblock Copolymers. Chem. Mater. 2017, 29, 4675–4681. [Google Scholar] [CrossRef]
- Svensson, B.; Alexandridis, P.; Olsson, U. Self-Assembly of a Poly(Ethylene Oxide)/Poly(Propylene Oxide) Block Copolymer (Pluronic P104, (EO)27(PO)61(EO)27) in the Presence of Water and Xylene. J. Phys. Chem. B 1998, 102, 7541–7548. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Liu, H.; Chen, J. Hydration and Conformation of Temperature-Dependent Micellization of PEO−PPO−PEO Block Copolymers in Aqueous Solutions by FT-Raman. Langmuir 1999, 15, 2703–2708. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Ma, J.; Wang, J.; Chen, S.; Liu, H. Temperature-Dependent Aggregation and Disaggregation of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Block Copolymer in Aqueous Solution. J. Phys. Chem. B 2007, 111, 13217–13220. [Google Scholar] [CrossRef]
- Waton, G.; Michels, B.; Zana, R. Dynamics of Micelles of Polyethyleneoxide-Polypropyleneoxide-Polyethyleneoxide Block Copolymers in Aqueous Solutions. J. Colloid Interface Sci. 1999, 212, 593–596. [Google Scholar] [CrossRef]
- Kositza, M.J.; Bohne, C.; Alexandridis, P.; Hatton, T.A.; Holzwarth, J.F. Dynamics of Micro- and Macrophase Separation of Amphiphilic Block-Copolymers in Aqueous Solution. Macromolecules 1999, 32, 5539–5551. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Zhang, G. Mesoscopic Simulation on Phase Behavior of Pluronic P104 Aqueous Solution at Moderate Concentrations. J. Dispers. Sci. Technol. 2008, 29, 1331–1337. [Google Scholar] [CrossRef]
- Wen, X.G.; Verrall, R.E. Temperature Study of Sound Velocity and Volume-Related Specific Thermodynamic Properties of Aqueous Solutions of Poly(Ethylene Oxide)–Poly(Propylene Oxide)–Poly (Ethylene Oxide) Triblock Copolymers. J. Colloid Interface Sci. 1997, 196, 215–223. [Google Scholar] [CrossRef]
- Alexandridis, P.; Nivaggioli, T.; Hatton, T.A. Temperature Effects on Structural Properties of Pluroncc P104 and F108 PEO-PPO-PEO Block Copolymer Solutions. Langmuir 1995, 11, 1468–1476. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, S.; Xu, G.; Liu, C. Mesoscopic Simulation of the Phase Separation on Triblock Copolymer in Aqueous Solution. Acta Phys.-Chim. Sin. 2007, 23, 139–144. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, K.; Liang, X.; Liu, H.; Li, Y. Small Molecule-Mediated Self-Assembly Behaviors of Pluronic Block Copolymers in Aqueous Solution: Impact of Hydrogen Bonding on the Morphological Transition of Pluronic Micelles. Soft Matter. 2020, 16, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Solubilization of Hydrocarbons and Resulting Aggregate Shape Transitions in Aqueous Solutions of Pluronic® (PEO–PPO–PEO) Block Copolymers. Colloids Surf. B 1999, 16, 55–72. [Google Scholar] [CrossRef]
- Polat, H.; Kutluay, G.; Polat, M. Analysis of Dilution Induced Disintegration of Micellar Drug Carriers in the Presence of Inter and Intra Micellar Species. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124989. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.-H.; Huang, J.S. Light-Scattering Studies of Concentrated Copolymer Micellar Solutions. Macromolecules 1998, 31, 6226–6233. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, S.H.; Huang, J.S. Small-Angle Neutron Scattering Studies of the Structure and Interaction of Tri-Block Co-Polymer Micelles in Aqueous Solution. Phys. B Condens. Matter. 1997, 241, 1019–1021. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.-H.; Huang, J.S. Small-Angle Neutron Scattering Analysis of the Structure and Interaction of Triblock Copolymer Micelles in Aqueous Solution. Macromolecules 1998, 31, 2236–2244. [Google Scholar] [CrossRef]
- Jia, L.; Guo, C.; Yang, L.; Xiang, J.; Tang, Y.; Liu, C.; Liu, H. Mechanism of PEO–PPO–PEO Micellization in Aqueous Solutions Studied by Two-Dimensional Correlation FTIR Spectroscopy. J. Colloid Interface Sci. 2010, 345, 332–337. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Liu, H. Melt, Hydration, and Micellization of the PEO–PPO–PEO Block Copolymer Studied by FTIR Spectroscopy. J. Colloid Interface Sci. 2002, 251, 417–423. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Wang, J.; Chen, J. Conformational Structure of Triblock Copolymers by FT-Raman and FTIR Spectroscopy. J. Colloid Interface Sci. 1999, 209, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Nivaggioli, T.; Alexandridis, P.; Hatton, T.A.; Yekta, A.; Winnik, M.A. Fluorescence Probe Studies of Pluronic Copolymer Solutions as a Function of Temperature. Langmuir 1995, 11, 730–737. [Google Scholar] [CrossRef]
- Holland, R.J.; Parker, E.J.; Guiney, K.; Zeld, F.R. Fluorescence Probe Studies of Ethylene Oxide/Propylene Oxide Block Copolymers in Aqueous Solution. J. Phys. Chem. 1995, 99, 11981–11988. [Google Scholar] [CrossRef]
- Hosseini Nejad, H. Mobility of Small Molecules in PEO-PPO-PEO Triblock Copolymer (F127 and P104) Hydrogels. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2021. [Google Scholar]
- Thompson, A.L.; Love, B.J. Thermodynamic Properties of Aqueous PEO–PPO–PEO Micelles of Varying Hydrophilicity with Added Cisplatin Determined by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2017, 127, 1583–1592. [Google Scholar] [CrossRef]
- Khimani, M.; Verma, G.; Kumar, S.; Hassan, P.A.; Aswal, V.K.; Bahadur, P. PH Induced Tuning of Size, Charge and Viscoelastic Behavior of Aqueous Micellar Solution of Pluronic® P104–Anthranilic Acid Mixtures: A Scattering, Rheology and NMR Study. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 202–210. [Google Scholar] [CrossRef]
- Stickler, M.; Panke, D.; Wunderlich, W. Solution Properties of Poly(Methyl Methacrylate) in Methyl Methacrylate, 1. Viscosities from the Dilute to the Concentrated Solution Regime. Die Makromol. Chem. 1987, 188, 2651–2664. [Google Scholar] [CrossRef]
- Álvarez-Ramírez, J.G.; Fernández, V.V.A.; Macías, E.R.; Rharbi, Y.; Taboada, P.; Gámez-Corrales, R.; Puig, J.E.; Soltero, J.F.A. Phase Behavior of the Pluronic P103/Water System in the Dilute and Semi-Dilute Regimes. J. Colloid Interface Sci. 2009, 333, 655–662. [Google Scholar] [CrossRef]
- Aeberhardt, K.; de Laumer, J.-Y.S.; Bouquerand, P.-E.; Normand, V. Ultrasonic Wave Spectroscopy Study of Sugar Oligomers and Polysaccharides in Aqueous Solutions: The Hydration Length Concept. Int. J. Biol. Macromol. 2005, 36, 275–282. [Google Scholar] [CrossRef]
- Bravo-Anaya, L.M.; Fierro-Castro, C.; Rharbi, Y.; Martínez, J.F.A.S. Triblock Copolymer P104 Detailed Behavior through a Density, Sound Velocity and DLS Study. AIP Conf. Proc. 2014, 1599, 481–484. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. A Record Nine Different Phases (Four Cubic, Two Hexagonal, and One Lamellar Lyotropic Liquid Crystalline and Two Micellar Solutions) in a Ternary Isothermal System of an Amphiphilic Block Copolymer and Selective Solvents (Water and Oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Tavano, L.; Nicoletta, F.P.; Trombino, S.; Cassano, R.; Picci, N. Liquid Crystalline Pluronic 105 Pharmacogels as Drug Delivery Systems: Preparation, Characterization, and in Vitro Transdermal Release. J. Drug Target. 2010, 18, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.L.; Phillips, R.J.; Cotts, P.M.; Dungan, S.R. Light Scattering Study on the Effect of Polymer Composition on the Structural Properties of PEO–PPO–PEO Micelles. J. Colloid Interface Sci. 1997, 191, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Verrall, R.E. Apparent Molar Volume and Adiabatic Compressibility Studies of Aqueous Solutions of Some Drug Compounds at 25 °C. Can. J. Chem. 1989, 67, 727–735. [Google Scholar] [CrossRef]
- Chi, Y.; Xu, S.; Xu, X.; Cao, Y.; Dong, J. Studies of Relationship between Polymer Structure and Hydration Environment in Amphiphilic Polytartaramides. J. Polym. Sci. B Polym. Phys. 2017, 55, 138–145. [Google Scholar] [CrossRef]
- Mata, J.P.; Majhi, P.R.; Guo, C.; Liu, H.Z.; Bahadur, P. Concentration, Temperature, and Salt-Induced Micellization of a Triblock Copolymer Pluronic L64 in Aqueous Media. J. Colloid Interface Sci. 2005, 292, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, J.; Liu, H. FTIR Spectroscopic Investigation of Effects of Temperature and Concentration on PEO−PPO−PEO Block Copolymer Properties in Aqueous Solutions. Macromolecules 2002, 35, 6426–6431. [Google Scholar] [CrossRef]
- Su, Y.; Liu, H.; Wang, J.; Chen, J. Study of Salt Effects on the Micellization of PEO−PPO−PEO Block Copolymer in Aqueous Solution by FTIR Spectroscopy. Langmuir 2002, 18, 865–871. [Google Scholar] [CrossRef]
- Sandler, S.I. Chemical, Biochemical, and Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Desai, P.R.; Jain, N.J.; Sharma, R.K.; Bahadur, P. Effect of Additives on the Micellization of PEO/PPO/PEO Block Copolymer F127 in Aqueous Solution. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 57–69. [Google Scholar] [CrossRef]
- Bharatiya, B.; Ghosh, G.; Bahadur, P.; Mata, J. The Effects of Salts and Ionic Surfactants on the Micellar Structure of Tri-Block Copolymer PEO-PPO-PEO in Aqueous Solution. J. Dispers. Sci. Technol. 2008, 29, 696–701. [Google Scholar] [CrossRef]
- Khimani, M.; Rao, U.; Bahadur, P.; Bahadur, P. Calorimetric and Scattering Studies on Micellization of Pluronics in Aqueous Solutions: Effect of the Size of Hydrophilic PEO End Blocks, Temperature, and Added Salt. J. Dispers. Sci. Technol. 2014, 35, 1599–1610. [Google Scholar] [CrossRef]
- Mazer, N.A.; Benedek, G.B.; Carey, M.C. An Investigation of the Micellar Phase of Sodium Dodecyl Sulfate in Aqueous Sodium Chloride Solutions Using Quasielastic Light Scattering Spectroscopy. J. Phys. Chem. C 1976, 80, 1075–1085. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Size- and Shape-Controlled Synthesis of Colloidal Gold through Autoreduction of the Auric Cation by Poly(Ethylene Oxide)–Poly(Propylene Oxide) Block Copolymers in Aqueous Solutions at Ambient Conditions. Nanotechnology 2005, 16, S344–S353. [Google Scholar] [CrossRef] [PubMed]
- Bokov, N.A. Experimental Investigation of the Influence of a Temperature Gradient on the Intensity of Light Scattering in Borate Melts. Glass Phys. Chem. 2010, 36, 158–165. [Google Scholar] [CrossRef]
- Landazuri, G.; Fernandez, V.V.A.; Soltero, J.F.A.; Rharbi, Y. Kinetics of the Sphere-to-Rod like Micelle Transition in a Pluronic Triblock Copolymer. J. Phys. Chem. B 2012, 116, 11720–11727. [Google Scholar] [CrossRef]
- Perrin, F. Mouvement Brownien d’un ellipsoide (II). Rotation libre et dépolarisation des fluorescences. Translation et diffusion de molecules ellipsoidales. J. Phys. Radium 1936, 7, 1–11. [Google Scholar] [CrossRef]
- Willis, S.A.; Dennis, G.R.; Zheng, G.; Price, W.S. Hydrodynamic Size and Scaling Relations for Linear and 4 Arm Star PVAc Studied Using PGSE NMR. J. Mol. Liq. 2010, 156, 45–51. [Google Scholar] [CrossRef]
- Thomas, H.G.; Lomakin, A.; Blankschtein, D.; Benedek, G.B. Growth of Mixed Nonionic Micelles. Langmuir 1997, 13, 209–218. [Google Scholar] [CrossRef]
- Doll, T.A.P.F.; Raman, S.; Dey, R.; Burkhard, P. Nanoscale Assemblies and Their Biomedical Applications. J. R. Soc. Interface 2013, 10, 20120740. [Google Scholar] [CrossRef]
- Kjøniksen, A.-L.; Zhu, K.; Pamies, R.; Nyström, B. Temperature-Induced Formation and Contraction of Micelle-Like Aggregates in Aqueous Solutions of Thermoresponsive Short-Chain Copolymers. J. Phys. Chem. B 2008, 112, 3294–3299. [Google Scholar] [CrossRef]
- Zhu, K.; Pamies, R.; Kjøniksen, A.-L.; Nyström, B. Temperature-Induced Intermicellization of “Hairy” and “Crew-Cut” Micelles in an Aqueous Solution of a Thermoresponsive Copolymer. Langmuir 2008, 24, 14227–14233. [Google Scholar] [CrossRef]
- Guo, L.; Luijten, E. Reversible gel formation of triblock copolymers studied by molecular dynamics simulation. J. Polym. Sci. B Polym. Phys. 2005, 43, 959–969. [Google Scholar] [CrossRef]
- Chamradová, I.; Vojtová, L.; Michlovská, L.; Poláček, P.; Jančář, J. Rheological Properties of Functionalised Thermosensitive Copolymers for Injectable Applications in Medicine. Chem. Pap. 2012, 66, 977–980. [Google Scholar] [CrossRef]
- Vermonden, T.; Besseling, N.A.M.; van Steenbergen, M.J.; Hennink, W.E. Rheological Studies of Thermosensitive Triblock Copolymer Hydrogels. Langmuir 2006, 22, 10180–10184. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Castelletto, V.; Hamley, I.W.; English, R.J.; Mingvanish, W. SANS and Rheology Study of Aqueous Solutions and Gels Containing Highly Swollen Diblock Copolymer Micelles. Langmuir 2003, 19, 3229–3235. [Google Scholar] [CrossRef]
- Kelarakis, A.; Castelletto, V.; Chaibundit, C.; Fundin, J.; Havredaki, V.; Hamley, I.W.; Booth, C. Rheology and Structures of Aqueous Gels of Triblock(Oxyethylene/Oxybutylene/Oxyethylene) Copolymers with Lengthy Oxyethylene Blocks. Langmuir 2001, 17, 4232–4239. [Google Scholar] [CrossRef]
- Wang, C.; Hashimoto, K.; Tamate, R.; Kokubo, H.; Morishima, K.; Li, X.; Shibayama, M.; Lu, F.; Nakanishi, T.; Watanabe, M. Viscoelastic Change of Block Copolymer Ion Gels in a Photo-Switchable Azobenzene Ionic Liquid Triggered by Light. Chem. Commun. 2019, 55, 1710–1713. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric Drug Delivery System Based on Pluronics for Cancer Treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Rahdar, A.; Kazemi, S.; Askari, F. Pluronic as nano-carier platform for drug delivery systems. Nanomed. Res. J. 2018, 3, 174–179. [Google Scholar] [CrossRef]
- Naharros-Molinero, A.; Caballo-González, M.Á.; de la Mata, F.J.; García-Gallego, S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics 2022, 14, 2628. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Prasanthan, P.; Kishore, N. Self-assemblies of pluronic micelles in partitioning of anticancer drugs and effectiveness of this system towards target protein. RSC Adv. 2021, 11, 22057–22069. [Google Scholar] [CrossRef] [PubMed]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug Delivery Systems Based on Pluronic Micelles with Antimicrobial Activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef] [PubMed]












| CP104 (wt.%) | AI × 104 (mPa·s) | BI (kJ/mol) | AIII × 104 (mPa·s) | BIII (kJ/mol) |
|---|---|---|---|---|
| 1 | 6.20 ± 0.4 | 2213 ± 24 | 47.80 ± 4.2 | 1443 ± 240 |
| 2 | 4.60 ± 0.2 | 2309 ± 12 | 19.90 ± 0.8 | 1852 ± 12 |
| 3 | 2.30 ± 0.2 | 2718 ± 180 | 23.30 ± 8.8 | 1864 ± 144 |
| 5 | 2.60 ± 0.4 | 2610 ± 60 | 13.90 ± 1.9 | 2092 ± 96 |
| 8 | 0.51 ± 0.14 | 3187 ± 60 | 25.80 ± 4.3 | 2104 ± 132 |
| 10 | 0.79 ± 0.01 | 3079 ± 84 | 1.60 ± 0.8 | 2730 ± 276 |
| 15 | 0.46 ± 0.01 | 3199 ± 265 | 0.12 ± 0.06 | 4017 ± 120 |
| 20 | 0.62 ± 0.05 | 3512 ± 24 | 0.08 ± 1 × 10−4 | 4438 ± 12 |
| CMT (°C) | MGT (°C) | CPT (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CP104 (wt.%) | η | dρs/dT | dUs/dT | dnH/dt | Turb. | Rheometry | dρs/dT | dUs/dT | dnH/dt | Rheometry | |
| 1 | 24 | 24 | 22 | 25 | 24.5 | 24 | - | - | 51 | 62 | 75 |
| 3 | 21 | 22 | 21.5 | 23.5 | 22.5 | - | - | - | 50 | - | - |
| 5 | 20 | 20 | 20 | 19.5 | 21.4 | 19 | 60 | 75 | |||
| 7 | - | 19 | 19 | 19 | - | - | |||||
| 8 | 19 | 18.5 | 18.5 | 20 | 20 | - | 53 | 51 | 51 | -- | -- |
| 10 | 18 | 17.6 | 17.5 | 19 | 19 | 17 | 56 | 51.5 | 51 | 58 | 75 |
| 15 | 16 | 15.3 | 15.2 | 16.5 | 16.7 | 16 | 51 | 51.5 | 50 | 59 | 76 |
| 20 | 15 | 13 | 12.8 | 14 | 14.3 | 11 | 51 | 52 | 50 | 59 | 75 |
| Region | exp (A) | ΔEDH (KJ/mol) | R2 |
|---|---|---|---|
| I | 5.4500 ± 0.86 | 10.8 ± 0.4 | 0.9741 |
| II | 1.6700 × 10−7 ± 8 × 10−8 | 53.9 ± 1.2 | 0.9944 |
| III | 0.055 ± 0.014 | 20.9 ± 0.7 | 0.9862 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Ochoa, E.B.; Bravo-Anaya, L.M.; Vaca-López, R.; Landázuri-Gómez, G.; Rosales-Rivera, L.C.; Diaz-Vidal, T.; Carvajal, F.; Macías-Balleza, E.R.; Rharbi, Y.; Soltero-Martínez, J.F.A. Structural Behavior of Amphiphilic Triblock Copolymer P104/Water System. Polymers 2023, 15, 2551. https://doi.org/10.3390/polym15112551
Figueroa-Ochoa EB, Bravo-Anaya LM, Vaca-López R, Landázuri-Gómez G, Rosales-Rivera LC, Diaz-Vidal T, Carvajal F, Macías-Balleza ER, Rharbi Y, Soltero-Martínez JFA. Structural Behavior of Amphiphilic Triblock Copolymer P104/Water System. Polymers. 2023; 15(11):2551. https://doi.org/10.3390/polym15112551
Chicago/Turabian StyleFigueroa-Ochoa, Edgar Benjamín, Lourdes Mónica Bravo-Anaya, Ricardo Vaca-López, Gabriel Landázuri-Gómez, Luis Carlos Rosales-Rivera, Tania Diaz-Vidal, Francisco Carvajal, Emma Rebeca Macías-Balleza, Yahya Rharbi, and J. Félix Armando Soltero-Martínez. 2023. "Structural Behavior of Amphiphilic Triblock Copolymer P104/Water System" Polymers 15, no. 11: 2551. https://doi.org/10.3390/polym15112551