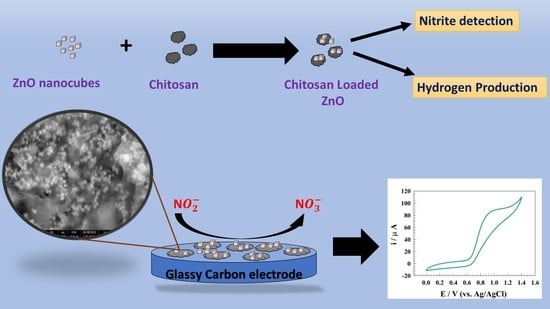
Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO Nanoparticles
2.2. Preparation of Zn-Chit Composite
2.3. Fabrication of Electrode
3. Result & Discussion
3.1. Catalyst Characterization
3.2. Zn-Chit Composite for Nitrite Detection
3.3. Zn-Chit Composite for Hydrogen Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Gundert-Remy, Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar]
- Das, J.K.; Pradhan, B. Study on influence of nitrite and phosphate based inhibiting admixtures on chloride interaction, rebar corrosion, and microstructure of concrete subjected to different chloride exposures. J. Build. Eng. 2022, 50, 104192. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Li, J.; Ren, W.; Dou, X. High-performance fluorescent and colorimetric dual-mode nitrite sensor boosted by a versatile coumarin probe equipped with diazotization-coupling reaction-sites. Sens. Actuators B Chem. 2023, 379, 133261. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, X.; Zu, B.; Dou, X. A March to Shape Optical Artificial Olfactory System toward Ultrasensitive Detection of Improvised Explosives. Adv. Photon-Res. 2022, 3, 2200006. [Google Scholar] [CrossRef]
- Yang, R.; Lin, Y.; Yang, J.; He, L.; Tian, Y.; Hou, X.; Zheng, C. Headspace Solid-Phase Microextraction Following Chemical Vapor Generation for Ultrasensitive, Matrix Effect-Free Detection of Nitrite by Microplasma Optical Emission Spectrometry. Anal. Chem. 2021, 93, 6972–6979. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, R.; Dong, C.; Cheng, F.; Guo, Y. Sensitive electrochemical sensor for nitrite ions based on rose-like AuNPs/MoS2/graphene composite. Biosens. Bioelectron. 2019, 142, 111529. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Chen, J.; Zhu, Y.; Hu, K.; Ma, Q.; Zuo, Y. A novel propylene glycol alginate gel based colorimetric tube for rapid detection of nitrite in pickled vegetables. Food Chem. 2022, 373, 131678. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, Z.; Dong, W.; Liu, Y.; Song, S.; Hu, Q.; Shuang, S.; Dong, C.; Gong, X. Intelligently design primary aromatic amines derived carbon dots for optical dual-mode and smartphone imaging detection of nitrite based on specific diazo coupling. J. Hazard. Mater. 2022, 430, 128393. [Google Scholar] [CrossRef]
- Chen, J.; Pang, S.; He, L.; Nugen, S.R. Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2016, 85, 726–733. [Google Scholar] [CrossRef]
- Büldt, A.; Karst, U. Determination of Nitrite in Waters by Microplate Fluorescence Spectroscopy and HPLC with Fluorescence Detection. Anal. Chem. 1999, 71, 3003–3007. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Elamrani, M.; Chtaini, A.; Isaad, J.; Belkhanchi, H.; Ziat, Y. Investigation on square wave and cyclic voltammetry approaches of the Pb2+, Cd2+, Co2+ and Hg2+ in tap water of Beni Mellal city (Morocco), Desalin. Water Treat. 2022, 280, 251–261. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Laghlimi, C.; Moutcine, A.; Chtaini, A.; Isaad, J.; Soufi, A.; Ziat, Y.; Amhamdi, H.; Belkhanchi, H. Recent advances in electrochemical sensors and biosensors for monitoring drugs and metabolites in pharmaceutical and biological samples. ADMET DMPK 2023. [Google Scholar] [CrossRef]
- Ifguis, O.; Moutcine, A.; Laghlimi, C.; Ziat, Y.; Bouhdadi, R.; Chtaini, A.; Moubarik, A.; Mbarki, M. Biopolymer-Modified Carbon Paste Electrode for the Electrochemical Detection of Pb(II) in Water. J. Anal. Methods Chem. 2022, 2022, 5348246. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, M.A.; Medany, S.S.; Fadlallah, S.A.; El-Sherif, R.M.; Hassan, S.S. Novel Self-assembly Pd(II)-Schiff Base Complex Modified Glassy Carbon Electrode for Electrochemical Detection of Paracetamol. Electrocatalysis 2022, 13, 598–610. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Competition between enzymatic and non-enzymatic electrochemical determination of cholesterol. J. Electroanal. Chem. 2023, 930, 117169. [Google Scholar] [CrossRef]
- Salih, E.; Mekawy, M.; Hassan, R.Y.A.; El-Sherbiny, I.M. Synthesis, characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J. Nanostruct. Chem. 2016, 6, 137–144. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An Electrochemical Glucose Sensor Based on Zinc Oxide Nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Pandikumar, A.; Yusoff, N.; Huang, N.M.; Lim, H.N. Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim. Acta 2015, 182, 1113–1122. [Google Scholar] [CrossRef]
- Akbari, Z.; Montazerozohori, M.; Bruno, G.; Moulaee, K.; Neri, G. Development of a novel electrochemical nitrite sensor based on Zn-Schiff base complexes. Appl. Organomet. Chem. 2022, 36, e6610. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Khaledi, H.; Pandikumar, A.; Rameshkumar, P.; Huang, N.M.; Arifin, Z.; Mazhar, M. Nitrite ion sensing properties of ZnTiO3–TiO2 composite thin films deposited from a zinc–titanium molecular complex. New J. Chem. 2015, 39, 7442–7452. [Google Scholar] [CrossRef]
- Annu; Raja, A.N. Recent development in chitosan-based electrochemical sensors and its sensing application. Int. J. Biol. Macromol. 2020, 164, 4231–4244. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, E.S. Characterization and properties of chitosan. Biotechnol. Biopolym. 2011, 91, 48–53. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Lamb, J.J.; Austbo, B. Hydrogen, Biomass, Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fang, Z.; Smith, R.L.; Qi, X. Production of Hydrogen from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Santhanam, K.S.V.; Press, R.J.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Sherif, S.A.; Goswami, D.Y.; Stefanakos, E.K.L.; Steinfeld, A. Handbook of Hydrogen Energy; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Vayssieres, L. On Solar Hydrogen and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lee, S.; Speight, J.G.; Loyalka, S.K. Handbook of Alternative Fuel Technologies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Yang, Y.; Mao, B.; Gong, G.; Li, D.; Liu, Y.; Cao, W.; Xing, L.; Zeng, J.; Shi, W.; Yuan, S. In-situ growth of Zn–AgIn5S8 quantum dots on g-C3N4 towards 0D/2D heterostructured photocatalysts with enhanced hydrogen production. Int. J. Hydrog. Energy 2019, 44, 15882–15891. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Trari, M. Photocatalytic hydrogen production from suspension of spinel powders AMn2O4 (A = Cu and Zn). Int. J. Hydrogen Energy 2002, 27, 357–362. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Zhang, L.; Fan, K. Controllable design of Zn-Ni-P on g-C3N4 for efficient photocatalytic hydrogen production. Chin. J. Catal. 2019, 40, 390–402. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Nafady, A.; Mohamed, S.K.; Medany, S.S. Facile green synthesis of Ag/carbon nanotubes composite for efficient water splitting applications. Synth. Met. 2023, 294, 117310. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.H.B.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef]
- Khalil, K.D.; Bashal, A.H.; Khalafalla, M.; Zaki, A.A. Synthesis, structural, dielectric and optical properties of chitosan-MgO nanocomposite. J. Taibah Univ. Sci. 2020, 14, 975–983. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, M.R.; Gafur, M.A.; Masum, S.M. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Lillo, L.; Pérez, J.; Muñoz, I.; Cabello, G.; Caro, C.; Lamilla, C.; Becerra, J. Production of exopolysaccharides by a submerged culture of an entomopathogenic fungus, Metarhizium anisopliae. Rev. Latinoam. Quím. 2014, 42, 70–76. [Google Scholar]
- Georgieva, V.; Zvezdova, D.; Vlaev, L.T. Non-isothermal kinetics of thermal degradation of chitosan. Chem. Central J. 2012, 6, 81. [Google Scholar] [CrossRef]
- Ye, D.; Luo, L.; Ding, Y.; Chen, Q.; Liu, X. A novel nitrite sensor based on graphene/polypyrrole/chitosan nanocomposite modified glassy carbon electrode. Analyst 2011, 136, 4563–4569. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef]
- Rao, H.; Liu, Y.; Zhong, J.; Zhang, Z.; Zhao, X.; Liu, X.; Jiang, Y.; Zou, P.; Wang, X.; Wang, Y. Gold Nanoparticle/Chitosan@N,S Co-doped Multiwalled Carbon Nanotubes Sensor: Fabrication, Characterization, and Electrochemical Detection of Catechol and Nitrite. ACS Sustain. Chem. Eng. 2017, 5, 10926–10939. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Systematic DFT studies of CO-Tolerance and CO oxidation on Cu-doped Ni surfaces. J. Mol. Graph. Model. 2023, 118, 108343. [Google Scholar] [CrossRef]
- Saad, B.; Bari, F.; Saleh, M.I.; Ahmad, K.; Talib, M.K.M. Simultaneous determination of preservatives (benzoic acid, sorbic acid, methylparaben and propylparaben) in foodstuffs using high-performance liquid chromatography. J. Chromatogr. A 2005, 1073, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, N.; Zhang, Y.; Ye, Z.; Yang, Y. Construction of La2O3-CeO2 Composites Modified Glassy Carbon Electrode as a Novel Electrochemical Sensor for Sensitive Detection of Nitrite. Chem. Lett. 2022, 51, 435–439. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Q.; Li, J.; Hong, C.; Zhao, Z.; Xu, H.; Hu, J. Synthesis and enhanced electrochemical properties of AuNPs@MoS2/rGO hybrid structures for highly sensitive nitrite detection. Microchem. J. 2022, 172, 106904. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Zhe, T.; Li, M.; Li, F.; Li, R.; Bai, F.; Bu, T.; Jia, P.; Wang, L. Integrating electrochemical sensor based on MoO3/Co3O4 heterostructure for highly sensitive sensing of nitrite in sausages and water. Food Chem. 2022, 367, 130666. [Google Scholar] [CrossRef]
- Heli, H.; Eskandari, I.; Sattarahmady, N.; Moosavi-Movahedi, A.A. Cobalt nanoflowers: Synthesis, characterization and derivatization to cobalt hexacyanoferrate—Electrocatalytic oxidation and determination of sulfite and nitrite. Electrochim. Acta 2012, 77, 294–301. [Google Scholar] [CrossRef]
- Afkhami, A.; Madrakian, T.; Ghaedi, H.; Khanmohammadi, H. Construction of a chemically modified electrode for the selective determination of nitrite and nitrate ions based on a new nanocomposite. Electrochim. Acta 2012, 66, 255–264. [Google Scholar] [CrossRef]
- Salagare, S.; Adarakatti, P.S.; Venkataramanappa, Y.; Almalki, A.S.A. Electrochemical nitrite sensing employing palladium oxide–reduced graphene oxide (PdO-RGO) nanocomposites: Application to food and environmental samples. Ionics 2022, 28, 927–938. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Sherif, R.M.A.; Hassan, H.K.; Hefnawy, M.A.; Galal, A. Conducting Polymer-Mixed Oxide Composite Electrocatalyst for Enhanced Urea Oxidation. J. Electrochem. Soc. 2018, 165, J3310–J3317. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Synergistic effect of Cu-doped NiO for enhancing urea electrooxidation: Comparative electrochemical and DFT studies. J. Alloy. Compd. 2021, 896, 162857. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. NiO-MnOx/Polyaniline/Graphite Electrodes for Urea Electrocatalysis: Synergetic Effect between Polymorphs of MnOx and NiO. Chemistryselect 2022, 7, e202103735. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; El-Bagoury, N.; Fadlallah, S.A. High-performance IN738 superalloy derived from turbine blade waste for efficient ethanol, ethylene glycol, and urea electrooxidation. J. Appl. Electrochem. 2023, 1–12. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Yen, Y.-K.; Yan, W.-M.; Huang, G.-W. Laser-scribed Graphene Electrodes Functionalized with Nafion/Fe3O4 Nanohybrids for the Ultrasensitive Detection of Neurotoxin Drug Clioquinol. ACS Omega 2022, 7, 15936–15950. [Google Scholar] [CrossRef]
- Tulli, F.; Zanini, V.I.P.; Fernández, J.M.; Martino, D.M.; De Mishima, B.A.L.; Borsarelli, C.D. Influence of Electrostatic Interactions Induced via a Nanocomposite Film onto a Glassy Carbon Electrode Used for Highly Selective and Sensitive Ascorbic Acid Detection. J. Electrochem. Soc. 2019, 166, B742–B747. [Google Scholar] [CrossRef]
- Butt, T.M.; Janjua, N.K.; Mujtaba, A.; Zaman, S.A.; Ansir, R.; Rafique, A.; Sumreen, P.; Mukhtar, M.; Pervaiz, M.; Yaqub, A.; et al. B-Site Doping in Lanthanum Cerate Nanomaterials for Water Electrocatalysis. J. Electrochem. Soc. 2020, 167, 026503. [Google Scholar] [CrossRef]
- Madej, M.; Matoga, D.; Skaźnik, K.; Porada, R.; Baś, B.; Kochana, J. A voltammetric sensor based on mixed proton-electron conducting composite including metal-organic framework JUK-2 for determination of citalopram. Microchim. Acta 2021, 188, 184. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, L.; Yang, L.; Ye, B. Study the voltammetric behavior of 10-Hydroxycamptothecin and its sensitive determination at electrochemically reduced graphene oxide modified glassy carbon electrode. Arab. J. Chem. 2019, 12, 2732–2739. [Google Scholar] [CrossRef]
- Malode, S.J.; Shetti, N.P.; Reddy, K.R. Highly sensitive electrochemical assay for selective detection of Aminotriazole based on TiO2/poly (CTAB) modified sensor. Environ. Technol. Innov. 2021, 21, 101222. [Google Scholar] [CrossRef]
- Huang, D.; Wu, H.; Zhu, Y.; Su, H.; Zhang, H.; Sheng, L.; Liu, Z.; Xu, H.; Song, C. Sensitive determination of anticancer drug methotrexate using graphite oxide-nafion modified glassy carbon electrode. Int. J. Electrochem. Sci. 2019, 14, 3792–3804. [Google Scholar] [CrossRef]
- Bornaei, M.; Khajehsharifi, H.; Shahrokhian, S.; Sheydaei, O.; Zarnegarian, A. Differential pulse voltammetric quantitation of kynurenic acid in human plasma using carbon-paste electrode modified with metal-organic frameworks. Mater. Chem. Phys. 2023, 295, 127016. [Google Scholar] [CrossRef]
- Laghlimi, C.; Ziat, Y.; Moutcine, A.; Hammi, M.; Zarhri, Z.; Ifguis, O.; Chtaini, A. A new sensor based on graphite carbon paste modified by an organic molecule for efficient determination of heavy metals in drinking water. Chem. Data Collect. 2021, 31, 100595. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; Hefnawy, M.A. Lanthanum nickel oxide nano-perovskite decorated carbon nanotubes/poly(aniline) composite for effective electrochemical oxidation of urea. J. Electroanal. Chem. 2020, 862, 114009. [Google Scholar] [CrossRef]
- Galal, A.; Atta, N.F.; Hefnawy, M.A. Voltammetry study of electrocatalytic activity of lanthanum nickel perovskite nanoclusters-based composite catalyst for effective oxidation of urea in alkaline medium. Synth. Met. 2020, 266, 116372. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Nickel-manganese double hydroxide mixed with reduced graphene oxide electrocatalyst for efficient ethylene glycol electrooxidation and hydrogen evolution reaction. Synth. Met. 2021, 282, 116959. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Zhang, J.; Cui, B.; Fang, Y. Determination of nitrite in food based on its sensitizing effect on cathodic electrochemiluminescence of conductive PTH-DPP films. Food Chem. 2022, 397, 133760. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Wu, J.; Liu, Y.; Geng, D.; Meng, X. Understanding the high-electrocatalytic performance of two-dimensional MoS2 nanosheets and their composite materials. J. Mater. Chem. A 2017, 5, 24540–24563. [Google Scholar] [CrossRef]
- Eliwa, A.S.; Hefnawy, M.A.; Medany, S.S.; Deghadi, R.G.; Hosny, W.M.; Mohamed, G.G. Ultrasonic-assisted synthesis of nickel metal-organic framework for efficient urea removal and water splitting applications. Synth. Met. 2023, 294, 117309. [Google Scholar] [CrossRef]
- Gawad, S.A.; Nasr, A.; Fekry, A.M.; Filippov, L.O. Electrochemical and hydrogen evolution behaviour of a novel nano-cobalt/nano-chitosan composite coating on a surgical 316L stainless steel alloy as an implant. Int. J. Hydrogen Energy 2021, 46, 18233–18241. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.; Jia, L.; Tan, X.; Chen, Y.; Huang, Q.; Shao, B.; Yu, T. Visible light driven hydrogen evolution using external and confined CdS: Effect of chitosan on carriers separation. Appl. Catal. B Environ. 2020, 277, 119152. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, J.; Huang, Y.; Qian, Q.; Luo, Y.; Xue, H.; Yang, S. Pt-chitosan-TiO2 for efficient photocatalytic hydrogen evolution via ligand-to-metal charge transfer mechanism under visible light. Molecules 2022, 27, 4673. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. Green synthesis of NiO/Fe3O4@chitosan composite catalyst based on graphite for urea electro-oxidation. Mater. Chem. Phys. 2022, 290, 126603. [Google Scholar] [CrossRef]











| Electrode | Linear Detection Range (µM) | Limit of Detection (µM) | Method | Reference |
|---|---|---|---|---|
| CeO2/La2O3 | 0.25 to 4000 | 0.015 | Amperometry | [49] |
| Au-MoS2@RGO | 0.2 to 2600 | 0.038 | Amperometry | [50] |
| GC/PANI/NiOnF | 1–500 | 0.064 | Amperometry | [51] |
| MoO3-Co3O4 | 0.3125 to 4514 | 0.075 | Amperometry | [52] |
| Cobalt-NFs | 100 to 2150 | 1.2 | Amperometry | [53] |
| SiO2-Fe3O4 | 0.72–110 | 0.74 | Amperometry | [54] |
| PdO@RGO | 10 to 1500 | 10.14 | differential pulse voltammetry | [55] |
| GC/ZnO | 1 to 150 | 0.689 | Amperometry | This work |
| GC/Zn-Chitosan | 1 to 150 | 0.402 | Amperometry | This work |
| Electrode | Rs | R1 | Q1 | R2 | Q2 | ||
|---|---|---|---|---|---|---|---|
| Ohm | Ohm | Y0 | N | Ohm | Y0 | m | |
| GC/ZnO | 27.238 | 2480.8 | 0.00013008 | 0.68866 | - | - | - |
| GC/Zn-Chitosan. | 43.505 | 640 | 0.002161 | 0.43496 | 1192.3 | 0.0000921 | 0.86971 |
| Sample | Added (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|
| Milk | 20 | 19 | 95 |
| 80 | 82 | 102 | |
| 150 | 146 | 97 | |
| 230 | 235 | 102 | |
| 300 | 297 | 99 |
| Electrode | Rs | Rc | Q1 | R2 | Q2 | ||
|---|---|---|---|---|---|---|---|
| Ohm | Ohm | Y0 | N | Ohm | Y0 | m | |
| GC/ZnO | 8.16 | 109.5 | 0.00046257 | 0.61097 | 216.49 | 0.0008012 | 0.79496 |
| GC/ZnO-Chit | 7.65 | 41.04 | 0.0010375 | 0.55337 | 110.9 | 0.0009921 | 0.80568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kadhi, N.S.; Hefnawy, M.A.; S. Nafee, S.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications. Polymers 2023, 15, 2357. https://doi.org/10.3390/polym15102357
Al-Kadhi NS, Hefnawy MA, S. Nafee S, Alamro FS, Pashameah RA, Ahmed HA, Medany SS. Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications. Polymers. 2023; 15(10):2357. https://doi.org/10.3390/polym15102357
Chicago/Turabian StyleAl-Kadhi, Nada S., Mahmoud A. Hefnawy, Sherif S. Nafee, Fowzia S. Alamro, Rami Adel Pashameah, Hoda A. Ahmed, and Shymaa S. Medany. 2023. "Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications" Polymers 15, no. 10: 2357. https://doi.org/10.3390/polym15102357