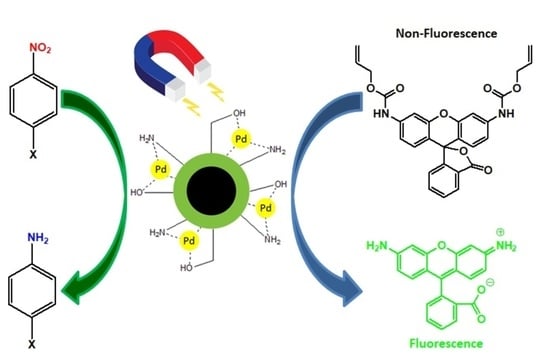
Palladium Nanoparticles on Chitosan-Coated Superparamagnetic Manganese Ferrite: A Biocompatible Heterogeneous Catalyst for Nitroarene Reduction and Allyl Carbamate Deprotection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pd-Chit@MnFe2O4
2.2.1. Preparation of MnFe2O4 Nanoparticles
2.2.2. Preparation of Chitosan-Coated MnFe2O4
2.2.3. Preparation of Magnetic-Supported Pd Catalyst
2.2.4. Preparation of Rhodamine 110
2.3. Catalyst Characterization
2.3.1. Structural Analysis
2.3.2. Elemental Analysis
2.3.3. Magnetic Properties
2.3.4. Catalytic Activity
Catalytic Reduction of Nitroarene Compounds and Reusability
Deprotection of Bis-allyloxycarbonyl Rhodamine 110 with and without Thiophenol
Stability and Cytotoxicity of Pd-Chit@MnFe2O4
3. Results and Discussion
3.1. Structural Analysis
3.2. Elemental Analysis
3.3. Magnetic Properties
3.4. Catalytic Activity
3.4.1. Catalytic Reduction of Nitroarenes and Reusability
3.4.2. Palladium-Induced Allyl Carbamate Deprotection
3.5. Cytotoxic Assay
3.6. BET Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaturvedi, S.; Dave, P.N.; Shah, N. Applications of nano-catalyst in new era. J. Saudi Chem. Soc. 2012, 16, 30N7–325. [Google Scholar] [CrossRef] [Green Version]
- Kotha, S.S.; Sharma, N.; Sekar, G. An Efficient, Stable and Reusable Palladium Nanocatalyst: Chemoselective Reduction of Aldehydes with Molecular Hydrogen in Water. Adv. Synth. Catal. 2016, 358, 1694–1698. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; El-Sayed, M.A. Enhancing Catalytic Efficiency of Hollow Palladium Nanoparticles by Photothermal Heating of Gold Nanoparticles Added to the Cavity: Palladium–Gold Nanorattles. ChemCatChem 2014, 6, 3540–3546. [Google Scholar] [CrossRef]
- Hu, C.; Yang, C.; Wang, X.; Wang, X.; Zhen, S.; Zhan, L.; Huang, C.; Li, Y. Rapid and facile synthesis of Au nanoparticle-decorated porous MOFs for the efficient reduction of 4-nitrophenol. Sep. Purif. Technol. 2022, 300, 121801. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Mirmohammadi, S.S.; Valadi, K.; Maleki, A.; Shalan, A.E. Convenient conversion of hazardous nitrobenzene derivatives to aniline analogues by Ag nanoparticles, stabilized on a naturally magnetic pumice/chitosan substrate. RSC Adv. 2020, 10, 43670–43681. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Shin, J.Y.; Song, C.E.; Lee, S.-G. Palladium nanoparticles supported onto ionic carbon nanotubes as robust recyclable catalysts in an ionic liquid. Chem. Commun. 2008, 8, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Guarnizo, A.; Angurell, I.; Muller, G.; Llorca, J.; Seco, M.; Rossell, O.; Rossell, M.D. Highly water-dispersible magnetite-supported Pd nanoparticles and single atoms as excellent catalysts for Suzuki and hydrogenation reactions. RSC Adv. 2016, 6, 68675–68684. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Ranjan, P.; Bera, P.; Ghosh, P.; Mandal, S.K. Anchored palladium nanoparticles onto single walled carbon nanotubes: Efficient recyclable catalyst for N-containing heterocycles. RSC Adv. 2012, 2, 7523–7533. [Google Scholar] [CrossRef]
- Shin, J.Y.; Lee, B.S.; Jung, Y.; Kim, S.J.; Lee, S.-G. Palladium nanoparticles captured onto spherical silica particles using a urea cross-linked imidazolium molecular band. Chem. Commun. 2007, 48, 5238–5240. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, P.; Werth, C.J.; Strathmann, T.J. Palladium nanoparticles encapsulated in core–shell silica: A structured hydrogenation catalyst with enhanced activity for reduction of oxyanion water pollutants. ACS Catal. 2014, 4, 3551–3559. [Google Scholar] [CrossRef]
- Huang, X.H.; Moon, B.K.; Byeon, S.J.; Heo, M.S.; Kim, I. Palladium nanoparticles decorated mesoporous carbon spheres as catalyst for reduction of 4-nitrophenol. J. Nanosci. Nanotechnol. 2014, 14, 8771–8776. [Google Scholar] [CrossRef] [PubMed]
- Siamaki, A.R.; Lin, Y.; Woodberry, K.; Connell, J.W.; Gupton, B.F. Palladium nanoparticles supported on carbon nanotubes from solventless preparations: Versatile catalysts for ligand-free Suzuki cross coupling reactions. J. Mater. Chem. A 2013, 1, 12909–12918. [Google Scholar] [CrossRef]
- Liew, K.H.; Loh, P.L.; Juan, J.C.; Yarmo, M.A.; Yusop, R.M. QuadraPure-supported palladium nanocatalysts for microwave-promoted Suzuki cross-coupling reaction under aerobic condition. Sci. World J. 2014, 2014, 796196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, K.H.; Samad, W.Z.; Nordin, N.; Loh, P.L.; Juan, J.C.; Yarmo, M.A.; Yahaya, B.H.; Yusop, R.M. Preparation and characterization of HypoGel-supported Pd nanocatalysts for Suzuki reaction under mild conditions. Chin. J. Catal. 2015, 36, 771–777. [Google Scholar] [CrossRef]
- Najman, R.; Cho, J.K.; Coffey, A.F.; Davies, J.W.; Bradley, M. Entangled palladium nanoparticles in resin plugs. Chem. Commun. 2007, 47, 5031–5033. [Google Scholar] [CrossRef] [PubMed]
- Sargin, I.; Baran, T.; Arslan, G. Environmental remediation by chitosan-carbon nanotube supported palladium nanoparticles: Conversion of toxic nitroarenes into aromatic amines, degradation of dye pollutants and green synthesis of biaryls. Sep. Purif. Technol. 2020, 247, 116987. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Jaleh, B.; Baran, T.; Varma, R.S. Efficient degradation of environmental contaminants using Pd-RGO nanocomposite as a retrievable catalyst. Clean Technol. Environ. Policy 2020, 22, 325–335. [Google Scholar] [CrossRef]
- Luo, W.; Luo, K.; Yang, Y.; Lin, X.; Li, P.; Wen, Y. N-maleyl chitosan-supported palladium catalyst for Heck coupling reaction and reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129852. [Google Scholar] [CrossRef]
- Akbayrak, S.; Kaya, M.; Volkan, M.; Özkar, S. Palladium (0) nanoparticles supported on silica-coated cobalt ferrite: A highly active, magnetically isolable and reusable catalyst for hydrolytic dehydrogenation of ammonia borane. Appl. Catal. B Environ. 2014, 147, 387–393. [Google Scholar] [CrossRef]
- Qiu, Y.; Ma, Z.; Hu, P. Environmentally benign magnetic chitosan/Fe3O4 composites as reductant and stabilizer for anchoring Au NPs and their catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2014, 2, 13471–13478. [Google Scholar] [CrossRef]
- Rahimi, J.; Taheri-Ledari, R.; Niksefat, M.; Maleki, A. Enhanced reduction of nitrobenzene derivatives: Effective strategy executed by Fe3O4/PVA-10%Ag as a versatile hybrid nanocatalyst. Catal. Commun. 2020, 134, 105850. [Google Scholar] [CrossRef]
- Baykal, A.; Karaoglu, E.; Sözeri, H.; Uysal, E.; Toprak, M.S. Synthesis and characterization of high catalytic activity magnetic Fe3O4 supported Pd nanocatalyst. J. Supercond. Nov. Magn. 2013, 26, 165–171. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Rahimi, J.; Maleki, A.; Shalan, A.E. Ultrasound-assisted diversion of nitrobenzene derivatives to their aniline equivalents through a heterogeneous magnetic Ag/Fe3O4-IT nanocomposite catalyst. New J. Chem. 2020, 44, 19827–19835. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Asgharnasl, S.; Mehraeen, S.; Amiri-Khamakani, Z.; Maleki, A. Guanidinylated SBA-15/Fe3O4 mesoporous nanocomposite as an efficient catalyst for the synthesis of pyranopyrazole derivatives. Sci. Rep. 2021, 11, 19852. [Google Scholar] [CrossRef]
- Bahrami, S.; Hassanzadeh-Afruzi, F.; Maleki, A. Synthesis and characterization of a novel and green rod-like magnetic ZnS/CuFe2O4/agar organometallic hybrid catalyst for the synthesis of biologically-active 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives. Appl. Organomet. Chem. 2020, 34, e5949. [Google Scholar] [CrossRef]
- Fernandes, C.; Pereira, C.; Fernandez-Garcia, M.P.; Pereira, A.M.; Guedes, A.; Fernandez-Pacheco, R.; Ibarra, A.; Ibarra, M.R.; Araujo, J.P.; Freire, C. Tailored design of CoxMn1−xFe2O4 nanoferrites: A new route for dual control of size and magnetic properties. J. Mater. Chem. C 2014, 2, 5818–5828. [Google Scholar] [CrossRef]
- Xu, W.-H.; Wang, L.; Wang, J.; Sheng, G.-P.; Liu, J.-H.; Yu, H.-Q.; Huang, X.-J. Superparamagnetic mesoporous ferrite nanocrystal clusters for efficient removal of arsenite from water. CrystEngComm 2013, 15, 7895–7903. [Google Scholar] [CrossRef]
- Rocha, M.; Fernandes, C.; Pereira, C.; Rebelo, S.L.; Pereira, M.F.; Freire, C. Gold-supported magnetically recyclable nanocatalysts: A sustainable solution for the reduction of 4-nitrophenol in water. Rsc Adv. 2015, 5, 5131–5141. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.e.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Iqbal, Y.; Bae, H.; Rhee, I.; Hong, S. Magnetic heating of silica-coated manganese ferrite nanoparticles. J. Magn. Magn. Mater. 2016, 409, 80–86. [Google Scholar] [CrossRef]
- Rufato, K.B.; Galdino, J.P.; Ody, K.S.; Pereira, A.G.; Corradini, E.; Martins, A.F.; Paulino, A.T.; Fajardo, A.R.; Aouada, F.A.; La Porta, F.A. Hydrogels based on chitosan and chitosan derivatives for biomedical applications. In Hydrogels-Smart Materials for Biomedical Applications; IntechOpen: London, UK, 2018. [Google Scholar]
- de Oliveira Arias, J.L.; Schneider, A.; Batista-Andrade, J.A.; Vieira, A.A.; Caldas, S.S.; Primel, E.G. Chitosan from shrimp shells: A renewable sorbent applied to the clean-up step of the QuEChERS method in order to determine multi-residues of veterinary drugs in different types of milk. Food Chem. 2018, 240, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.-Y.; Den, W. Chitosan as a natural polymer for heterogeneous catalysts support: A short review on its applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef] [Green Version]
- Dabbawala, A.A.; Sudheesh, N.; Bajaj, H.C. Palladium supported on chitosan as a recyclable and selective catalyst for the synthesis of 2-phenyl ethanol. Dalton Trans. 2012, 41, 2910–2917. [Google Scholar] [CrossRef]
- Baig, R.N.; Nadagouda, M.N.; Varma, R.S. Ruthenium on chitosan: A recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem. 2014, 16, 2122–2127. [Google Scholar] [CrossRef]
- Baig, R.N.; Varma, R.S. Copper on chitosan: A recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- de Souza, J.F.; da Silva, G.T.; Fajardo, A.R. Chitosan-based film supported copper nanoparticles: A potential and reusable catalyst for the reduction of aromatic nitro compounds. Carbohydr. Polym. 2017, 161, 187–196. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Yan, N. Shell biorefinery: Dream or reality? Chem.–A Eur. J. 2016, 22, 13402–13421. [Google Scholar] [CrossRef]
- Thorat, N.; Otari, S.; Patil, R.; Bohara, R.; Yadav, H.; Koli, V.; Chaurasia, A.; Ningthoujam, R. Synthesis, characterization and biocompatibility of chitosan functionalized superparamagnetic nanoparticles for heat activated curing of cancer cells. Dalton Trans. 2014, 43, 17343–17351. [Google Scholar] [CrossRef]
- Streu, C.; Meggers, E. Ruthenium-induced allylcarbamate cleavage in living cells. Angew. Chem. Int. Ed. 2006, 45, 5645–5648. [Google Scholar] [CrossRef]
- Yusop, R.M.; Unciti-Broceta, A.; Johansson, E.; Sánchez-Martín, R.M.; Bradley, M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239–243. [Google Scholar] [CrossRef]
- Kamalzare, M.; Ahghari, M.R.; Bayat, M.; Maleki, A. Fe3O4@chitosan-tannic acid bionanocomposite as a novel nanocatalyst for the synthesis of pyranopyrazoles. Sci. Rep. 2021, 11, 20021. [Google Scholar] [CrossRef] [PubMed]
- Kamalzare, M.; Bayat, M.; Maleki, A. Green and efficient three-component synthesis of 4H-pyran catalysed by CuFe2O4@starch as a magnetically recyclable bionanocatalyst. R. Soc. Open Sci. 2020, 7, 200385. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Intini, S.; Romanazzi, G.; Rizzuti, A.; Leonelli, C.; Piccinni, F.; Mastrorilli, P. Polymer supported palladium nanocrystals as efficient and recyclable catalyst for the reduction of nitroarenes to anilines under mild conditions in water. J. Mol. Catal. A Chem. 2014, 395, 307–314. [Google Scholar] [CrossRef]
- Vincent, T.; Peirano, F.; Guibal, E. Chitosan supported palladium catalyst. VI. Nitroaniline degradation. J. Appl. Polym. Sci. 2004, 94, 1634–1642. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour. Technol. 2013, 132, 374–377. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhu, Y.; Tian, G.; Wang, A. In situ generation of silver nanoparticles within crosslinked 3D guar gum networks for catalytic reduction. Int. J. Biol. Macromol. 2015, 73, 39–44. [Google Scholar] [CrossRef]
- Silambarasan, S.; Vangnai, A.S. Biodegradation of 4-nitroaniline by plant-growth promoting Acinetobacter sp. AVLB2 and toxicological analysis of its biodegradation metabolites. J. Hazard. Mater. 2016, 302, 426–436. [Google Scholar] [CrossRef]
- Shen, H.; Gao, J.; Wang, J. Assessment of toxicity of two nitroaromatic compounds in the freshwater fish Cyprinus carpio. Front. Environ. Sci. Eng. 2012, 6, 518–523. [Google Scholar] [CrossRef]
- Wu, W.; Liu, G.; Liang, S.; Chen, Y.; Shen, L.; Zheng, H.; Yuan, R.; Hou, Y.; Wu, L. Efficient visible-light-induced photocatalytic reduction of 4-nitroaniline to p-phenylenediamine over nanocrystalline PbBi2Nb2O9. J. Catal. 2012, 290, 13–17. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Singh, R.; Jana, S. Characterization of physical, thermal and spectroscopic properties of biofield energy treated p-phenylenediamine and p-toluidine. Environ. Anal. Toxicol. 2015, 5, 1–10. [Google Scholar]
- Ikarashi, Y.; Kaniwa, M.-A. Determination of p-Phenylenediamine and Related Antioxidants in Rubber Boots by High Performance Liquid Chromatography. Development of an Analytical Method for N-(1-Methylheptyl)-N′-pheny1-p-phenylenediamine. J. Health Sci. 2000, 46, 467–473. [Google Scholar] [CrossRef]
- Robert, A. Phenyleneand Toluenediamines. In Ullmann’s Encyclopedia of Industrial Chemistry; Verlag Chemie: Hoboken, NJ, USA, 2002. [Google Scholar]
- Beugelmans, R.; Neuville, L.; Bois-Choussy, M.; Chastanet, J.; Zhu, J. Palladium catalyzed reductive deprotection of alloc: Transprotection and peptide bond formation. Tetrahedron Lett. 1995, 36, 3129–3132. [Google Scholar] [CrossRef]
- Franco, D.; Duñach, E. New and mild allyl carbamate deprotection method catalyzed by electrogenerated nickel complexes. Tetrahedron Lett. 2000, 41, 7333–7336. [Google Scholar] [CrossRef]
- Vutukuri, D.R.; Bharathi, P.; Yu, Z.; Rajasekaran, K.; Tran, M.-H.; Thayumanavan, S. A mild deprotection strategy for allyl-protecting groups and its implications in sequence specific dendrimer synthesis. J. Org. Chem. 2003, 68, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández-García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.-M.; Araújo, J.o.P. Superparamagnetic MFe2O4 (M= Fe, Co, Mn) nanoparticles: Tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Liew, K.H.; Lee, T.K.; Yarmo, M.A.; Loh, K.S.; Peixoto, A.F.; Freire, C.; Yusop, R.M. Ruthenium Supported on Ionically Cross-linked Chitosan-Carrageenan Hybrid MnFe2O4 Catalysts for 4-Nitrophenol Reduction. Catalysts 2019, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S. Modification of chitosan for sorption of metal ions. J. Chem. Pharm. Res. 2015, 7, 49–55. [Google Scholar]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural characterisation of ZnO particles obtained by the emulsion precipitation method. J. Nanomater. 2012, 2012, 15. [Google Scholar] [CrossRef] [Green Version]
- Rivas, P.; Sagredo, V.; Rossi, F.; Pernechele, C.; Solzi, M.; Peña, O. Structural, magnetic, and optical characterization of $MnFe2O4nanoparticles synthesized via sol-Gel method. IEEE Trans. Magn. 2013, 49, 4568–4571. [Google Scholar] [CrossRef]
- Yang, L.-X.; Wang, F.; Meng, Y.-F.; Tang, Q.-H.; Liu, Z.-Q. Fabrication and characterization of manganese ferrite nanospheres as a magnetic adsorbent of chromium. J. Nanomater. 2013, 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, F.; Keßler, M.T.; Dohmen, S.; Singh, M.; Prechtl, M.H.; Mathur, S. Molecular palladium precursors for Pd0 nanoparticle preparation by microwave irradiation: Synthesis, structural characterization and catalytic activity. Eur. J. Inorg. Chem. 2012, 2012, 6027–6033. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Layek, S.; Pathak, D.D. Palladium nanoparticles immobilized on a magnetic chitosan-anchored Schiff base: Applications in Suzuki–Miyaura and Heck–Mizoroki coupling reactions. New J. Chem. 2017, 41, 5595–5604. [Google Scholar]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and dissolution study of chitosan freeze-dried systems for drug controlled release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Ling, C.; Peck, T.C.; Jia, H. Understanding the chemical state of palladium during the direct NO decomposition–influence of pretreatment environment and reaction temperature. RSC Adv. 2017, 7, 19645–19655. [Google Scholar] [CrossRef] [Green Version]
- Veisi, H.; Najafi, S.; Hemmati, S. Pd (II)/Pd (0) anchored to magnetic nanoparticles (Fe3O4) modified with biguanidine-chitosan polymer as a novel nanocatalyst for Suzuki-Miyaura coupling reactions. Int. J. Biol. Macromol. 2018, 113, 186–194. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Liu, Y.; Shang, N.; Wang, H.-J.; Wang, C.; Gao, Y. Palladium nanoparticles anchored on sustainable chitin for phenol hydrogenation to cyclohexanone. ACS Sustain. Chem. Eng. 2020, 8, 12304–12312. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, J.; Martinez-Hernandez, J.L.; Segura-Ceniceros, P.; Lopez, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases immobilization on chitosan-coated magnetic nanoparticles: Application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chang, P.R.; Li, Z.; Wang, H.; Liang, H.; Cao, X.; Chen, Y. Chitosan-coated cellulose/soy protein membranes with improved physical properties and hemocompatibility. BioResources 2011, 6, 1392–1413. [Google Scholar]
- Salah, T.A.; Mohammad, A.M.; Hassan, M.A.; El-Anadouli, B.E. Development of nano-hydroxyapatite/chitosan composite for cadmium ions removal in wastewater treatment. J. Taiwan Inst. Chem. Eng. 2014, 45, 1571–1577. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Cabanas-Polo, S.; Gao, H.; Gilabert, J.; Sanchez, E.; Roether, J.; Schubert, D.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of nanostructured-TiO2/chitosan composite coatings on stainless steel. RSC Adv. 2013, 3, 11247–11254. [Google Scholar] [CrossRef] [Green Version]
- Bujňáková, Z.; Dutková, E.; Kello, M.; Mojžiš, J.; Baláž, M.; Baláž, P.; Shpotyuk, O. Mechanochemistry of chitosan-coated zinc sulfide (ZnS) nanocrystals for bio-imaging applications. Nanoscale Res. Lett. 2017, 12, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sionkowska, A.; Płanecka, A. Preparation and characterization of silk fibroin/chitosan composite sponges for tissue engineering. J. Mol. Liq. 2013, 178, 5–14. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.; Friedrich, H.B. The current status of heterogeneous palladium catalysed Heck and Suzuki cross-coupling reactions. Molecules 2018, 23, 1676. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Beland, F.; Ciriminna, R.; Palmisano, G.; Cara, P.D. A new class of heterogeneous Pd catalysts for synthetic organic chemistry. Catal. Sci. Technol. 2011, 1, 736–739. [Google Scholar] [CrossRef]
- Rashad, M.M. Magnetic properties of nanocrystalline magnesium ferrite by co-precipitation assisted with ultrasound irradiation. J. Mater. Sci. 2007, 42, 5248–5255. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanoparticle Res. 2012, 14, 964. [Google Scholar] [CrossRef]
- Le, X.; Dong, Z.; Liu, Y.; Jin, Z.; Huy, T.-D.; Le, M.; Ma, J. Palladium nanoparticles immobilized on core–shell magnetic fibers as a highly efficient and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol and Suzuki coupling reactions. J. Mater. Chem. A 2014, 2, 19696–19706. [Google Scholar] [CrossRef]
- Babji, P.S.; Rao, V.L. Catalytic reduction of 4-Nitrophenol to 4-Aminophenol by using Fe2O3-Cu2O-TiO2 nanocomposite. Int. J. Chem. Stud. 2016, 4, 123–127. [Google Scholar]
- Lee, J.H.; Hong, S.K.; Ko, W.B. Reduction of 4-Nitrophenol Catalyzed by Platinum Nanoparticles Embedded into Carbon Nanocolloids. Asian J. Chem. 2011, 23, 2347–2350. [Google Scholar]
- Kurtan, U.; Amir, M.; Baykal, A. A Fe3O4@ Nico@ Ag nanocatalyst for the hydrogenation of nitroaromatics. Chin. J. Catal. 2015, 36, 705–711. [Google Scholar] [CrossRef]
- Noschese, A.; Buonerba, A.; Canton, P.; Milione, S.; Capacchione, C.; Grassi, A. Highly efficient and selective reduction of nitroarenes into anilines catalyzed by gold nanoparticles incarcerated in a nanoporous polymer matrix: Role of the polymeric support and insight into the reaction mechanism. J. Catal. 2016, 340, 30–40. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mandal, K.; Dasgupta, S. Hydrazine assisted catalytic hydrogenation of PNP to PAP by NixPd100−x nanocatalyst. RSC Adv. 2016, 6, 64364–64373. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, N.; Li, H.; Yao, Q.; Xu, F.; Fan, J.; Du, J.; Peng, X. Probing thiophenol pollutant in solutions and cells with BODIPY-based fluorescent probe. Ind. Eng. Chem. Res. 2017, 56, 9303–9309. [Google Scholar] [CrossRef]
- Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122. [Google Scholar] [CrossRef]










| Analyte | Mean Concentration (ppb) | Metal Loading (mmol/g) | Simplest Ratio |
|---|---|---|---|
| Palladium (Pd) | 912.75 | 0.21 | - |
| Manganese (Mn) | 7286.01 | 3.32 | 1 |
| Iron (Fe) | 13,523.70 | 6.05 | 1.8 |
| Nanomaterial | Magnetic Characterization | |||
|---|---|---|---|---|
| Ms (emu/g) | Hc (Oe) | Mr (emu/g) | Mr/Ms | |
| MnFe2O4 | 58.4 | 22.8 | 2.0 | 0.03 |
| Chit@MnFe2O4 | 38.2 | 22.5 | 1.2 | 0.03 |
| Pd-Chit@MnFe2O4 | 27.7 | 31.4 | 1.3 | 0.05 |
The chemical equation of nitroarene reduction: | |||
| Condition | Time (s) | k (min−1) | TOF (min−1) |
| (a) 4-NP reduction *: | |||
| 1.0 mg Pd-Chit@MnFe2O4 | 120 | 1.72 | 357.1 |
| 0.5 mg Pd−Chit@MnFe2O4 | 200 | 0.67 | 285.7 |
| 1.0 mg Chit@MnFe2O4 | 3600 | 0.012 | - |
| 1.0 mg MnFe2O4 | 3600 | 0.0048 | - |
| Without catalyst | 3 days | - | - |
| (b) 4-NA reduction *: | |||
| 0.5 mg Pd-Chit@MnFe2O4 | 150 | 1.42 | 571.4 |
| 0.5 mg Chit@MnFe2O4 | 600 | 0.36 | - |
| 0.5 mg MnFe2O4 | 720 | 0.26 | - |
| Without catalyst | 1 day | 0.000018 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebadi, M.; Asikin-Mijan, N.; Md. Jamil, M.S.; Iqbal, A.; Yousif, E.; Md Zain, A.R.; Aziz, T.H.T.; Rahimi Yusop, M. Palladium Nanoparticles on Chitosan-Coated Superparamagnetic Manganese Ferrite: A Biocompatible Heterogeneous Catalyst for Nitroarene Reduction and Allyl Carbamate Deprotection. Polymers 2023, 15, 232. https://doi.org/10.3390/polym15010232
Ebadi M, Asikin-Mijan N, Md. Jamil MS, Iqbal A, Yousif E, Md Zain AR, Aziz THT, Rahimi Yusop M. Palladium Nanoparticles on Chitosan-Coated Superparamagnetic Manganese Ferrite: A Biocompatible Heterogeneous Catalyst for Nitroarene Reduction and Allyl Carbamate Deprotection. Polymers. 2023; 15(1):232. https://doi.org/10.3390/polym15010232
Chicago/Turabian StyleEbadi, Mona, Nurul Asikin-Mijan, Mohd Suzeren Md. Jamil, Anwar Iqbal, Emad Yousif, Ahmad Rifqi Md Zain, Tengku Hasnan Tengku Aziz, and Muhammad Rahimi Yusop. 2023. "Palladium Nanoparticles on Chitosan-Coated Superparamagnetic Manganese Ferrite: A Biocompatible Heterogeneous Catalyst for Nitroarene Reduction and Allyl Carbamate Deprotection" Polymers 15, no. 1: 232. https://doi.org/10.3390/polym15010232