Effects of Peroxide Initiator on the Structure and Properties of Ultralow Loss Thermosetting Polyphenylene Oxide-Based Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
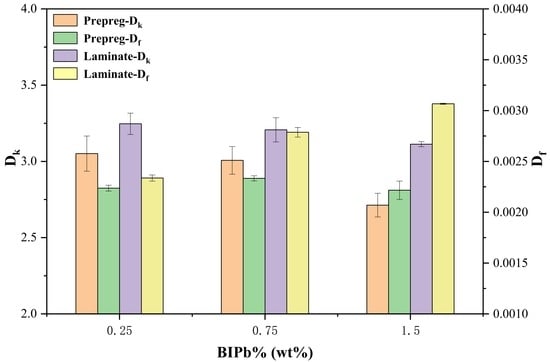
3. Results and Discussion
3.1. Chemical Structure of Cured Matrix
3.2. Unreacted TAIC in Laminates
3.3. Thermal-Mechanical Properties and Free Volume
3.4. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, J.G.; Buzzi, S.; Choi, W.; Hanly, S.V.; Lozano, A.; Soong, A.C.K.; Zhang, J.C. What Will 5G Be? IEEE J. Sel. Areas Commun. 2014, 32, 1065–1082. [Google Scholar] [CrossRef]
- Alsulami, M.M.; Akkari, N. The Role of 5G Wireless Networks in the Internet-of-Things (IoT). In Proceedings of the 2018 1st International Conference on Computer Applications Information Security (ICCAIS), Riyadh, Saudi Arabia, 4–6 April 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Tasaki, T.; Shiotani, A.; Tsuji, M.; Nakamura, T.; Yamaguchi, T. The Low Dk / Df Adhesives for High Frequency Printed Circuit Board Using the Novel Solvent Soluble Polyimide. In Proceedings of the 2015 10th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 21–23 October 2015; pp. 221–224. [Google Scholar] [CrossRef]
- Tseng, F.P.; Chuang, K.Y.; Liao, L.S.; Chiou, K.C. High Frequency Substrate Materials with Highly Thermal Resistance and Low Dielectric Properties. In Proceedings of the 2013 8th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 22–25 October 2013; pp. 109–112. [Google Scholar] [CrossRef]
- Xu, M.; Chen, S.; Li, X.; Zhu, S.; Ren, D.; Liu, X. Fabrication of Phthalonitrile-Based Copper-Clad Laminates and Their Application Properties: Thermo-Stability and Dielectric Properties. Adv. Ind. Eng. Polym. Res. 2020, 3, 194–201. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, J.; Liang, G.; Yuan, L. Methyl silsesquioxane/cyanate ester resin organic–inorganic hybrids with low dielectric constant. J. Appl. Polym. Sci. 2011, 121, 1004–1012. [Google Scholar] [CrossRef]
- Chao, H.S.I.; Whalen, J.M. Poly(2,6-Dimethyl-1,4-Phenylene Ether) (PPO®) Capping and Its Significance in the Preparation of PPO®/Epoxy Laminate. J. Appl. Polym. Sci. 1996, 59, 473–481. [Google Scholar] [CrossRef]
- Liang, G.; Meng, J.; Zhao, L. Synthesis of Styrene–Maleic Anhydride Random Copolymer and its Compatibilization to Poly(2,6-dimethyl-1,4-phenylene ether)/Brominated Epoxy Resin. Polym. Int. 2003, 52, 966–972. [Google Scholar] [CrossRef]
- Seike, Y.; Okude, Y.; Iwakura, I.; Chiba, I.; Ikeno, T.; Yamada, T. Synthesis of Polyphenylene Ether Derivatives: Estimation of Their Dielectric Constants. Macromol. Chem. Phys. 2003, 204, 1876–1881. [Google Scholar] [CrossRef]
- Krijgsman, J.; Feijen, J.; Gaymans, R.J. Segmented Copolymers of Uniform Tetra-Amide Units and Poly(Phenylene Oxide): 1. Synthesis and Thermal-Mechanical Properties. Polymer 2004, 45, 4677–4684. [Google Scholar] [CrossRef]
- Su, M.S. Composite Material, High-Frequency Circuit Substrate Made Therefrom and Making Method Thereof. U.S. Patent 9890276B2, 13 February 2018. [Google Scholar]
- Almen, G.R.; Wang, K.; Yuan, Q. Thermosetting Resin Composition Containing a Polyphenylene Ether and a Brominated Fire Retardant Compound. U.S. Patent 9243164B1, 26 January 2016. [Google Scholar]
- Fung, D.R.; Liao, T.C.; Chen, H.S.; Chen, C.L. High-Frequency Copper Foil Covered Substrated and Compound Material Used Therein. U.S. Patent 9243132B2, 26 January 2016. [Google Scholar]
- Almen, G.R. Thermosetting Resin Composition Containing a Polyphenylene Ether and a Brominated Fire Retardant Compound. U.S. Patent 9051465b1, 9 June 2015. [Google Scholar]
- Tokiwa, T.; Utsumi, T.; Endo, M. Curable Resin Composition. U.S. Patent 8703277B2, 22 April 2014. [Google Scholar]
- Yokoyama, D.; Saito, H.; Kitai, Y.; Fujiwara, H.; Inagaki, K. Developing Thermosetting Resin System with Low Molecular Weight PPE to Achieve Lower Dielectric Property. J. Netw. Polym. 2012, 33, 336–340. [Google Scholar] [CrossRef]
- Ishll, Y.; Kuroki, M.; Oda, H.; Arai, T.; Katayose, T. Novel Thermosettable Poly (Phenylene Ether). Synthesis, Blend, and Application to Copper Clad Laminates. Kobunshi Ronbunshu 1997, 54, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Gu, Z.C.; Tsai, Y.L.; Jeng, R.J.; Lin, C.H. Identification of the Reaction Mechanism between Phenyl Methacrylate and Epoxy and Its Application in Preparing Low-Dielectric Epoxy Thermosets with Flexibility. Polymer 2018, 140, 225–232. [Google Scholar] [CrossRef]
- Chen, C.H.; Jheng, J.K.; Juang, T.Y.; Abu-Omar, M.M.; Lin, C.H. Structure-Property Relationship of Vinyl-Terminated Oligo(2,6-Dimethyl-1,4-Phenylene Ether)s (OPEs): Seeking an OPE with Better Properties. Eur. Polym. J. 2019, 117, 94–104. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, K.W.; Lin, C.H.; Juang, T.Y. Low-Dissipation Thermosets Derived from Oligo(2,6-Dimethyl Phenylene Oxide)-Containing Benzoxazines. Polymers 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.; Zhang, Y.; Zhang, X.; Liu, L.; Zhang, H. Synthesis and Properties of Cyanate Mixed Resin Systems Modified by Polyphenylene Oxide for Production of High-Frequency Copper Clad Laminates. J. Mater. Sci. Mater. Electron. 2018, 29, 2831–2840. [Google Scholar] [CrossRef]
- Yang, M.C.; Higashihara, T.; Su, H.W.; Ueda, M.; Chen, W.C. Crosslinked Copolymer with Low Dielectric Constant and Dissipation Factor Based on Poly(2,6-Dimethylphenol-Co−2,6-Diphenylphenol) and a Crosslinker. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3218–3223. [Google Scholar] [CrossRef]
- Mo, J.; Xia, L.; Pan, P.; Shentu, B.; Weng, Z. Reactive Blend of Epoxy-Novolac Resin and Epoxide-Terminated Low-Molecular-Weight Poly(Phenylene Oxide). J. Appl. Polym. Sci. 2013, 127, 4879–4888. [Google Scholar] [CrossRef]
- Gao, R.; Gu, A.; Liang, G.; Dai, S.; Yuan, L. Properties and Origins of High-Performance Poly(Phenylene Oxide)/Cyanate Ester Resins for High-Frequency Copper-Clad Laminates. J. Appl. Polym. Sci. 2011, 121, 1675–1684. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hsu, S.W.; Wang, C.S. Low Dielectric and Flame-Retardant Properties of Thermosetting Redistributed Poly(Phenylene Oxide). J. Vinyl Addit. Technol. 2009, 15, 54–59. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hsu, S.W.; Wang, C.S. Low Dielectric Thermoset from Redistributed Poly(Phenylene Oxide). J. Macromol. Sci. Part A 2008, 45, 1047–1054. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hsu, S.W.; Chung, C.L.; Wang, C.S. Low Dielectric Epoxy Resins from Dicyclopentadiene-Containing Poly(Phenylene Oxide) Novolac Cured with Dicyclopentadiene Containing Epoxy. React. Funct. Polym. 2008, 68, 1185–1193. [Google Scholar] [CrossRef]
- Nunoshige, J.; Akahoshi, H.; Liao, Y.; Horiuchi, S.; Shibasaki, Y.; Ueda, M. Mechanical and Dielectric Properties of a New Polymer Blend Composed of 1,2-Bis(Vinylphenyl)Ethane and Thermosetting Poly(Phenylene Ether) Copolymer Obtained from 2,6-Dimethylphenol and 2-Allyl-6-Methylphenol. Polym. J. 2007, 39, 828–833. [Google Scholar] [CrossRef] [Green Version]
- Peters, E.N. Polyphenylene Ether Macromonomers. I. Property Enhancements in Thermoset Resins via Novel Telechelic Oligomers. In Proceedings of the Annual Technical Conference Proceedings (ANTEC 2007), Cincinnati, OH, USA, 6–11 May 2007; pp. 2125–2128. [Google Scholar]
- Peters, E.N.; Fisher, S.M.; Guo, H.; Howe, R.; Degonzague, C. Polyphenylene Ether Macromolecules. Ⅶ. Performance in t-Butly Styrene/Divinyl Benzene Resin System. In Proceedings of the Annual Technical Conference Proceedings (ANTEC 2010), Orlando, FL, USA, 16–20 May 2010; pp. 1858–1861. [Google Scholar]
- Peters, E.N.; Fisher, S.M.; Guo, H. PPE Macromonomers. Ⅸ. Performance Enhancements of Triallyl Isocyanurate Resins. In Proceedings of the Annual Technical Conference Proceedings (ANTEC 2011), Boston, MA, USA, 1–5 May 2011; Volume 3, pp. 2823–2826. [Google Scholar]
- Peters, E.N.; Jeevanath, M.; Fisher, S.M.; Guo, H. Polyphenylene Ether Macromonomer: Ⅹ. Vinyl Terminated Telechelic Macromers. In Proceedings of the Annual Technical Conference Proceedings (ANTEC 2011), Boston, MA, USA, 1–5 May 2011; pp. 2819–2822. [Google Scholar]
- Shieh, J.Y.; Yang, S.P.; Wu, M.F.; Wang, C.S. Synthesis and characterization of novel low-dielectric cyanate esters. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2589–2600. [Google Scholar] [CrossRef]
- Ahmad, Z. Polymer Dielectric Materials; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, Q. Novel Ferroelectric Polymers for High Energy Density and Low Loss Dielectrics. Macromolecules 2012, 45, 2937–2954. [Google Scholar] [CrossRef]
- Zhou, D.L.; Li, J.H.; Guo, Q.Y.; Lin, X.; Zhang, Q.; Chen, F.; Han, D.; Fu, Q. Polyhedral Oligomeric Silsesquioxanes Based Ultralow-k Materials: The Effect of Cage Size. Adv. Funct. Mater. 2021, 31, 2102074. [Google Scholar] [CrossRef]
- Luo, Y.; Jin, K.; He, C.; Wang, J.; Sun, J.; He, F.; Zhou, J.; Wang, Y.; Fang, Q. An Intrinsically Microporous Network Polymer with Good Dielectric Properties at High Frequency. Macromolecules 2016, 49, 7314–7321. [Google Scholar] [CrossRef]
- Dlubek, G.; Saarinen, K.; Fretwell, H.M. The Temperature Dependence of the Local Free Volume in Polyethylene and Polytetrafluoroethylene: A Positron Lifetime Study. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1513–1528. [Google Scholar] [CrossRef]
- Yu, J.; Huo, R.; Chao, W.; Wu, X.; Wang, G.; Jiang, P. Influence of Interface Structure on Dielectric Properties of Epoxy/Alumina Nanocomposites. Macromol. Res. 2012, 20, 816–826. [Google Scholar] [CrossRef]
- Siddabattuni, S.; Schuman, T.P.; Dogan, F. Improved Polymer Nanocomposite Dielectric Breakdown Performance through Barium Titanate to Epoxy Interface Control. Mater. Sci. Eng. B 2011, 176, 1422–1429. [Google Scholar] [CrossRef]
- Huang, X.; Iizuka, T.; Jiang, P.; Ohki, Y.; Tanaka, T. Role of Interface on the Thermal Conductivity of Highly Filled Dielectric Epoxy/AlN Composites. J. Phys. Chem. C 2012, 116, 13629–13639. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, J.; Gan, J.; Liang, L.; Wu, K.; Lu, M. Relationship between Crosslinking Structure and Low Dielectric Constant of Hydrophobic Epoxies Based on Substituted Biphenyl Mesogenic Units. RSC Adv. 2015, 5, 88014–88020. [Google Scholar] [CrossRef]
- Yang, D.; Tian, M.; Kang, H.; Dong, Y.; Liu, H.; Yu, Y.; Zhang, L. New Polyester Dielectric Elastomer with Large Actuated Strain at Low Electric Field. Mater. Lett. 2012, 76, 229–232. [Google Scholar] [CrossRef]
- Bakule, R.; Havranek, A. The Dependence of Dielectric Properties on Crosslink Density of Rubbers. Rubber Chem. Technol. 1978, 51, 72–80. [Google Scholar] [CrossRef]
- Wu, B.; Mao, X.; Wang, C.; Deng, T.; Li, R.; Xu, Y.; Tang, X. Different Organic Peroxides that Cure Low-k 1, 2-PB/SBS/EPDM Composites for High-Frequency Substrate. J. Vinyl Addit. Technol. 2020, 26, 524–535. [Google Scholar] [CrossRef]
- Walker, R.C.; Hamedi, H.; Woodward, W.H.H.; Rajagopalan, R.; Lanagan, M. Impacts of Crosslinking and Degassing on the Conductivity, Dielectric Loss, and Morphology of Low-Density Polyethylene and Crosslinked Polyethylene. In Broadband Dielectric Spectroscopy: A Modern Analytical Technique; American Chemical Society: Washington, DC, USA, 2021; pp. 239–260. [Google Scholar] [CrossRef]
- El Harfi, J.; Kingman, S.W.; Dimitrakis, G.; Robinson, J.P.; Irvine, D.J. Dielectric Properties of Free Radical Initiators Investigation of Thermal Decomposition Products. Ind. Eng. Chem. Res. 2012, 51, 15811–15820. [Google Scholar] [CrossRef]
- Yu, X.; Fang, Z.; Qin, Y.; Lu, D.; Liu, Q.; Luo, L.; Lin, Z.; Wang, K. FTIR and NMR Characterization of Thermosetting Methyl Methacrylate Terminated Poly(2,6-Dimethyl-1,4-Phenylene Oxide)—Triallyl Isocyanurate Copolymer. J. Polym. Res. 2021, 28, 272. [Google Scholar] [CrossRef]
- Yuki, K.; Hiroaki, F.; Hirosuke, S. Thermosetting Resin Composition, Prepreg, Metal Clad Laminate Plate, and Printed Wiring Board. U.S. Patent 9708468B2, 18 July 2017. [Google Scholar]
- Lin, H.; Wang, Z.; Zhong, D. Polyphenylene Ether Resin Composition. U.S. Patent 10167389B2, 1 January 2019. [Google Scholar]
- Thomas, A.K. Circuit Materials and Articles Formed Therefrom. U.S. Patent 9809690B2, 7 November 2017. [Google Scholar]
- Reig, F.B.; Adelantado, J.G.; Moreno, M.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Matsumoto, A.; Hirai, F.; Sumiyama, Y.; Aota, H.; Takayama, Y.; Kameyama, A.; Nakanishi, T. Further Discussion of Steric Effect on the Radical Polymerization of Triallyl Isocyanurate as Compared with Its Isomer Triallyl Cyanurate: Polymerization and Copolymerization of Corresponding Trimethallyl Compounds. Eur. Polym. J. 1999, 35, 195–199. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of Crosslink Density in Short Chain Networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Pan, X.; Webster, D.C. Impact of Structure and Functionality of Core Polyol in Highly Functional Biobased Epoxy Resins. Macromol. Rapid Commun. 2011, 32, 1324–1330. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and Mechanical Properties of Epoxy Acrylate/Methacrylates UV Cured Coatings. Prog. Org. Coat. 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Rusli, A.; Cook, W.; Liang, G.G. Allylic Monomers as Reactive Plasticizers of Polyphenylene Oxide. Part I: Uncured Systems. Eur. Polym. J. 2011, 47, 1775–1784. [Google Scholar] [CrossRef]
- Abaci, U.; Guney, H.Y.; Yilmazoglu, M. Plasticizer Effect on Dielectric Properties of Poly(Methyl Methacrylate)/Titanium Dioxide Composites. Polym. Polym. Compos. 2021, 29, 565–574. [Google Scholar] [CrossRef]
- Shelly, M.; Mathew, M.; Pradyumnan, P.P.; Francis, T. Dielectric and Thermal Stability Studies on High Density Polyethylene–Chitosan Composites Plasticized with Palm Oil. Mater. Today Proc. 2021, 46, 2742–2746. [Google Scholar] [CrossRef]














| Sample | SA9000:TAIC:MEK (Mass Ratio) | BIPb (wt% of mSA9000 + TAIC) |
|---|---|---|
| P/T-0.25 | 4:3:3 | 0.25 |
| P/T-0.50 | 4:3:3 | 0.50 |
| P/T-0.75 | 4:3:3 | 0.75 |
| P/T-1.00 | 4:3:3 | 1.00 |
| P/T-1.25 | 4:3:3 | 1.25 |
| P/T-1.50 | 4:3:3 | 1.50 |
| Sample | SA9000:TAIC:MEK (Mass Ratio) | BIPb (wt% of mSA9000 + TAIC) |
|---|---|---|
| P/T-0.25-L | 4:3:3 | 0.25 |
| P/T-0.75-L | 4:3:3 | 0.75 |
| P/T-1.50-L | 4:3:3 | 1.50 |
| P/T-0.00-L Powder (mg) | mTAIC (mg) | nTAIC (mmol) | n–C=C– (mmol) | APeak area-3086 |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 1.56 | 0.6686 | 0.0027 | 0.0081 | 0.48 |
| 2.87 | 1.2300 | 0.0049 | 0.0148 | 0.83 |
| 4.52 | 1.9371 | 0.0078 | 0.0233 | 1.78 |
| 5.99 | 2.5671 | 0.0103 | 0.0309 | 1.88 |
| 8.60 | 3.6857 | 0.0148 | 0.0444 | 3.72 |
| Sample | Weight (mg) | HPLC (mg) | wt% (in TAIC) | wt% (in Sample) | 1H-NMR (mg) | wt% (in TAIC) | wt% (in Sample) |
|---|---|---|---|---|---|---|---|
| P/T-0.25 | 4433.0 | 276.72 | 14.74% | 6.24% | 286.29 | 15.26% | 6.46% |
| P/T-1.50 | 3690.4 | 7.49 | 0.48% | 0.20% | 0 | 0 | 0 |
| BIPb% | Tg-tanδ/DMA (°C) | E’/Tg + 40 °C (MPa) | |
|---|---|---|---|
| 0.25 | 172.0 | 9.0 | 814.53 |
| 0.50 | 200.5 | 24.7 | 2076.97 |
| 0.75 | 213.0 | 37.7 | 3097.26 |
| 1.00 | 229.9 | 74.7 | 5937.05 |
| 1.25 | 231.6 | 77.3 | 6134.94 |
| 1.50 | 236.0 | 100.0 | 7876.82 |
| BIPb% | r3 (Å) | V3 (Å3) | I3 (%) | FFV (%) | |
|---|---|---|---|---|---|
| 0.25 | 2.19 | 3.02 | 115.03 | 40.1 | 6.9 |
| 0.50 | 2.29 | 3.10 | 125.03 | 38.5 | 7.2 |
| 0.75 | 2.33 | 3.14 | 129.06 | 38.4 | 7.4 |
| 1.00 | 2.44 | 3.22 | 140.62 | 36.8 | 7.6 |
| 1.25 | 2.51 | 3.28 | 147.27 | 33.0 | 8.0 |
| 1.50 | 2.57 | 3.32 | 153.42 | 31.5 | 8.5 |
| Prepreg Sample | Melt Viscosity (cP) |
|---|---|
| P/T-0.25-L | 10,739 |
| P/T-0.75-L | 20,884 |
| P/T-1.50-L | 23,454 |
| Prepreg Sample | Residual Rate of –C=C– in TAIC |
|---|---|
| P/T-0.25-L | 56.06% |
| P/T-0.75-L | 52.62% |
| P/T-1.50-L | 45.94% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Fang, Z.; Liu, Q.; Li, D.; Meng, Y.; Luo, C.; Wang, K.; Lin, Z. Effects of Peroxide Initiator on the Structure and Properties of Ultralow Loss Thermosetting Polyphenylene Oxide-Based Composite. Polymers 2022, 14, 1752. https://doi.org/10.3390/polym14091752
Yu X, Fang Z, Liu Q, Li D, Meng Y, Luo C, Wang K, Lin Z. Effects of Peroxide Initiator on the Structure and Properties of Ultralow Loss Thermosetting Polyphenylene Oxide-Based Composite. Polymers. 2022; 14(9):1752. https://doi.org/10.3390/polym14091752
Chicago/Turabian StyleYu, Xueyi, Zeming Fang, Qianfa Liu, Dan Li, Yundong Meng, Cheng Luo, Ke Wang, and Zhiyong Lin. 2022. "Effects of Peroxide Initiator on the Structure and Properties of Ultralow Loss Thermosetting Polyphenylene Oxide-Based Composite" Polymers 14, no. 9: 1752. https://doi.org/10.3390/polym14091752