Thermal Degradation of Photoluminescence Poly(9,9-dioctylfluorene) Solvent-Tuned Aggregate Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pristine PFO Films and Aggregated PFO Films
2.3. Thermal Degradation Test
2.4. Characterization
2.5. Calculation of β-Phase Fraction and Crystallinity Percentage and in PFO Films
3. Results and Discussion
3.1. Production of PFO Aggregate Films
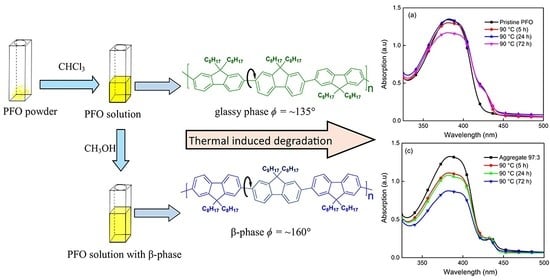
3.2. Thermal Induced Degradation of Aggregated PFO Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, K.; Kazuo, A. Helically assembled π-conjugated polymers with circularly polarized luminescence. Sci. Technol. Adv. Mater. 2014, 15, 044203. [Google Scholar] [CrossRef] [PubMed]
- Sekine, C.; Tsubata, Y.; Yamada, T.; Kitano, M.; Doi, S. Recent progress of high performance polymer OLED and OPV materials for organic printed electronics. Sci. Technol. Adv. Mater. 2014, 15, 034203. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Yin, C.R.; Lai, W.Y.; Fan, Q.L.; Huang, W. Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics. Prog. Polym. Sci. 2012, 37, 1192–1264. [Google Scholar] [CrossRef]
- Shi, W.X.; Liu, N.; Zhou, Y.M.; Cao, X.A. Effects of postannealing on the characteristics and reliability of polyfluorene organic light-emitting diodes. IEEE Trans. Electron Devices 2019, 66, 1057–1062. [Google Scholar] [CrossRef]
- Bradley, D.D.; Grell, M.; Long, X.; Mellor, J.; Grice, A.W.; Inbasekaran, M.; Woo, E.P. Influence of aggregation on the optical properties of a polyfluorene. In Optical Probes of Conjugated Polymers, Proceedings of the Optical Science, Engineering and Instrumentation ’97, San Diego, CA, USA, 27 July–1 August 1997; SPIE: Bellingham, WA, USA, 1997; Volume 3145, pp. 254–259. [Google Scholar] [CrossRef]
- Liang, J.; Yu, L.; Zhao, S.; Ying, L.; Liu, F.; Yang, W.; Peng, J.; Cao, Y. Improving efficiency and color purity of poly(9,9-dioctylfluorene) through addition of a high boiling-point solvent of 1-chloronaphthalene. Nanotechnology 2016, 27, 284001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Chi, L.; Hai, G.; Fang, Y.; Li, X.; Xia, R.; Huang, W.; Gu, E. An easy approach to control β-phase formation in PFO films for optimized emission properties. Molecules 2017, 22, 315. [Google Scholar] [CrossRef]
- Kobin, B.; Behren, S.; Braun-Cula, B.; Hecht, S. Photochemical Degradation of Various Bridge-Substituted Fluorene-Based Materials. J. Phys. Chem. A 2016, 120, 5474–5480. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, T.; White, J.M. On the origin of green emission in polyfluorene polymers: The roles of thermal oxidation degradation and crosslinking. Adv. Funct. Mater. 2004, 14, 783–790. [Google Scholar] [CrossRef]
- Grisorio, R.; Allegretta, G.; Mastrorilli, P.; Suranna, G.P. On the degradation process involving polyfluorenes and the factors governing their spectral stability. Macromolecules 2011, 44, 7977–7986. [Google Scholar] [CrossRef]
- Kobin, B.; Bianchi, F.; Halm, S.; Leistner, J.; Blumstengel, S.; Henneberger, F.; Hecht, S. Green emission in ladder-type quarterphenyl: Beyond the fluorenone-defect. Adv. Funct. Mater. 2014, 24, 7717–7727. [Google Scholar] [CrossRef]
- Sainova, D.; Neher, D.; Dobruchowska, E.; Luszczynska, B.; Glowacki, I.; Ulanski, J.; Nothofer, H.G.; Scherf, U. Thermoluminescence and electroluminescence of annealed polyfluorene layers. Chem. Phys. Lett. 2003, 371, 15–22. [Google Scholar] [CrossRef]
- Gamerith, S.; Gaal, M.; Romaner, L.; Nothofer, H.G.; Güntner, R.; de Freitas, P.S.; Scherf, U.; List, E.K.W. Comparison of thermal and electrical degradation effects in polyfluorenes. Synth. Met. 2003, 139, 855–858. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Z.; Hu, Q.; Lin, J.; Chen, Y.; Xie, L.; Lai, W.; Huang, W. Stable pure-blue polymer light-emitting devices based on β-phase poly(9,9-dioctylfluorene) induced by 1,2-dichloroethane. Appl. Phys. Express 2014, 7, 101601. [Google Scholar] [CrossRef]
- Becker, K.; Lupton, J.M. Dual species emission from single polyfluorene molecules: Signatures of stress-induced planarization of single polymer chains. J. Am. Chem. Soc. 2005, 127, 7306–7307. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Liu, C.Y.; Chang, C.H.; Chen, S.A. Self-dopant formation in poly(9,9-di-n-octylfluorene) via a dipping method for efficient and stable pure-blue electroluminescence. Adv. Mater. 2007, 19, 2574–2579. [Google Scholar] [CrossRef]
- Jiang, Y.; McNeill, J. Light-harvesting and amplified energy transfer in conjugated polymer nanoparticles. Chem. Rev. 2017, 117, 838–859. [Google Scholar] [CrossRef]
- Chang, M.; Lim, G.T.; Park, B.; Reichmanis, E. Control of molecular ordering, alignment, and charge transport in solution-processed conjugated polymer thin films. Polymers 2017, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Schulz, G.L.; Ludwigs, S. Controlled crystallization of conjugated polymer films from solution and solvent vapor for polymer electronics. Adv. Func. Mater. 2017, 27, 1603083. [Google Scholar] [CrossRef]
- Nakamura, T.; Vacha, M. Mechanically induced conformation change, fluorescence modulation, and mechanically assisted photodegradation in single nanoparticles of the conjugated polymer poly(9,9-dioctylfluorene). J. Phys. Chem. Lett. 2020, 11, 3103–3110. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Remarkably high miscibility of octa-substituted POSS with commodity conjugated polymers and molecular fillers for the improvement of homogeneities of polymer matrices. Polym. J. 2016, 48, 1133–1139. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhang, Q.; Yu, X.H.; Liu, J.G.; Han, Y.C. Increasing the Content of β Phase of Poly(9,9-dioctylfluorene) by Synergistically Controlling Solution Aggregation and Extending Film-forming Time. Chin. J. Polym. Sci. 2019, 37, 664–673. [Google Scholar] [CrossRef]
- Rahim, N.A.A.; Fujiki, M. Aggregation-induced scaffolding: Photoscissable helical polysilane generates circularly polarized luminescent polyfluorene. Polym. Chem. 2016, 7, 4618–4629. [Google Scholar] [CrossRef] [Green Version]
- Fujiki, M.; Yoshida, K.; Suzuki, N.; Rahim, N.A.A.; Abd Jalil, J. Tempo-spatial chirogenesis. Limonene-induced mirror symmetry breaking of Si–Si bond polymers during aggregation in chiral fluidic media. J. Photochem. Photobiol. A Chem. 2016, 331, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Perevedentsev, A.; Chander, N.; Kim, J.S.; Bradley, D.D. Spectroscopic properties of poly(9,9-dioctylfluorene) thin films possessing varied fractions of β-phase chain segments: Enhanced photoluminescence efficiency via conformation structuring. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Huang, X.; Sun, G.; Gu, C.; Lu, D.; Ma, Y. Study of β phase and chains aggregation degrees in poly(9,9-dioctylfluorene) (PFO) solution. J. Phys. Chem. C 2012, 116, 7993–7999. [Google Scholar] [CrossRef]
- Traiphol, R.; Sanguansat, P.; Srikhirin, T.; Kerdcharoen, T.; Osotchan, T. Spectroscopic study of photophysical change in collapsed coils of conjugated polymers: Effects of solvent and temperature. Macromolecules 2006, 39, 1165–1172. [Google Scholar] [CrossRef]
- Bearzotti, A.; Macagnano, A.; Pantalei, S.; Zampetti, E.; Venditti, I.; Fratoddi, I.; Russo, M.V. Alcohol vapor sensory properties of nanostructured conjugated polymers. J. Phys. Condens. Matter 2008, 20, 474207. [Google Scholar] [CrossRef]
- Gupta, S. Solvation chemistry through synergism: Static and dynamic features of: N-amyl alcohol-chloroform binary solvent mixture. RSC Adv. 2016, 6, 99306–99313. [Google Scholar] [CrossRef]
- Chabinyc, M.L. X-ray scattering from films of semiconducting polymers. Polym. Rev. 2008, 48, 463–492. [Google Scholar] [CrossRef]
- Kwon, E.H.; Kim, G.W.; Kim, M.; Park, Y.D. Effect of Alcohol Polarity on the Aggregation and Film-Forming Behaviors of Poly(3-hexylthiophene). ACS Appl. Polym. Mater. 2020, 2, 2980–2986. [Google Scholar] [CrossRef]
- O’Carroll, D.; Lacopino, D.; O’Riordan, A.; Lovera, P.; O’Connor, É.; O’Brien, G.A.; Redmond, G. Poly(9,9-dioctylfluorene) nanowires with pronounced β-phase morphology: Synthesis, characterization, and optical properties. Adv. Mater. 2008, 20, 42–48. [Google Scholar] [CrossRef]
- Ariu, M.; Sims, M.; Rahn, M.D.; Hill, J.; Fox, A.M.; Lidzey, D.G.; Oda, M.; Cabanillas-Gonzalez, J.; Bradley, D.D.C. Exciton migration in β-phase poly(9,9-dioctylfluorene). Phys. Rev. B 2003, 67, 195333. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kinoshita, K.; Niwa, A.; Nagase, T.; Naito, H. Photoluminescence Properties of Polymorphic Modifications of Low Molecular Weight Poly(3-hexylthiophene). Nanoscale Res. Lett. 2017, 12, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.; Liu, Y.; Li, T.; Li, X.; Liu, B.; Lu, D. Quantitative study on β-phase heredity based on poly(9, 9-dioctylfluorene) from solutions to films and the effect on hole mobility. J. Phys. Chem. C 2016, 120, 27820–27828. [Google Scholar] [CrossRef]
- Li, T.; He, Q.; Guan, Y.; Liao, J.; He, Y.; Luo, X.; Cao, W.; Cui, Z.; Jia, S.; Liu, A.; et al. Influence of molecular weight and the change of solvent solubility on β conformation and chains condensed state structure for poly(9,9-dioctylfluorene) (PFO) in solution. Polymer 2022, 240, 124471. [Google Scholar] [CrossRef]
- Nakamura, T.; Sharma, D.K.; Hirata, S.; Vacha, M. Intrachain aggregates as the origin of green emission in polyfluorene studied on ensemble single-chain level. J. Phys. Chem. C 2018, 122, 8137–8146. [Google Scholar] [CrossRef]
- Liu, L.; Lu, P.; Xie, Z.; Wang, H.; Tang, S.; Wang, Z.; Zhang, W.; Ma, Y. Role of nonemissive quenchers for the green emission in polyfluorene. J. Phys. Chem. B 2007, 111, 10639–10644. [Google Scholar] [CrossRef]
- Jen, T.H.; Wang, K.K.; Chen, S.A. Effect of thermal stability on performance of β-phase poly(9,9-di-n-octylfluorene) in deep blue electroluminescence. Polymer 2012, 53, 5850–5855. [Google Scholar] [CrossRef]
- Guha, S.; Rice, J.D.; Yau, T.T.; Martin, C.M.; Chandrasekhar, M.; Chandrasekhar, H.R.; Guentner, R.; De Freitas, P.S.; Scherf, U. Temperature-dependent photoluminescence of organic semiconductors with varying backbone conformation. Phys. Rev. B 2003, 67, 125204. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Su, A.G.; Su, C.H.; Chen, S.A. Crystalline Forms and Emission Behavior of Poly(9,9-di-n-octyl-2,7-fluorene). Macromolecules 2005, 38, 379–385. [Google Scholar] [CrossRef]
- Kawana, S.; Durrell, M.; Lu, J.; Macdonald, J.E.; Grell, M.; Bradley, D.D.C.; Jukes, P.C.; Jones, R.A.L.; Bennett, S.L. X-ray diffraction study of the structure of thin polyfluorene films. Polymer 2002, 43, 1907–1913. [Google Scholar] [CrossRef]
- Liem, H. A natural length scale for the glass transition of conjugated polymer film. J. Phys. Condens. Matter 2007, 19, 416106. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Su, A.C.; Chen, S.A. Noncrystalline Phases in Poly(9,9-di-n-octyl-2,7-fluorene). J. Phys. Chem. B 2005, 109, 10067–10072. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.; Formentin, P.; Martinez-Ferrero, E.; Pallarès, J.; Marsal, L.F. β-Phase Morphology in Ordered Poly(9,9-dioctylfluorene) Nanopillars by Template Wetting Method. Nanoscale Res. Lett. 2011, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliznyuk, V.N.; Carter, S.A.; Scott, J.C.; Klärner, G.; Miller, R.D.; Miller, D.C. Electrical and photoinduced degradation of polyfluorene based films and light-emitting devices. Macromolecules 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Kawamura, T.; Misaki, M.; Koshiba, Y.; Horie, S.; Kinashi, K.; Ishida, K.; Ueda, Y. Crystalline thin films of β-phase poly(9,9-dioctylfluorene). Thin Solid Films 2011, 519, 2247–2250. [Google Scholar] [CrossRef]
- Kuik, M.; Wetzelaer, G.J.A.; Laddé, J.G.; Nicolai, H.T.; Wildeman, J.; Sweelssen, J.; Blom, P.W. The effect of ketone defects on the charge transport and charge recombination in polyfluorenes. Adv. Funct. Mater. 2011, 21, 4502–4509. [Google Scholar] [CrossRef]
- Fujiki, M.; Okazaki, S.; Rahim, N.A.A.; Yamada, T.; Nomura, K. Synchronization in Non-Mirror-Symmetrical Chirogenesis: Non-Helical π-Conjugated Polymers with Helical Polysilane Copolymers in Co-Colloids. Symmetry 2021, 13, 594. [Google Scholar] [CrossRef]
- Yu, M.N.; Soleimaninejad, H.; Lin, J.Y.; Zuo, Z.Y.; Liu, B.; Bo, Y.F.; Bai, L.B.; Han, Y.M.; Smith, T.A.; Xu, M.; et al. Photophysical and fluorescence anisotropic behavior of polyfluorene β-conformation films. J. Phys. Chem. Lett. 2018, 9, 364–372. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, K.W.; Abdul Rahim, N.A.; Teh, P.L.; Abdul Hisam, N.S.; Alias, S.S. Thermal Degradation of Photoluminescence Poly(9,9-dioctylfluorene) Solvent-Tuned Aggregate Films. Polymers 2022, 14, 1615. https://doi.org/10.3390/polym14081615
Chew KW, Abdul Rahim NA, Teh PL, Abdul Hisam NS, Alias SS. Thermal Degradation of Photoluminescence Poly(9,9-dioctylfluorene) Solvent-Tuned Aggregate Films. Polymers. 2022; 14(8):1615. https://doi.org/10.3390/polym14081615
Chicago/Turabian StyleChew, Kang Wei, Nor Azura Abdul Rahim, Pei Leng Teh, Nurfatin Syafiqah Abdul Hisam, and Siti Salwa Alias. 2022. "Thermal Degradation of Photoluminescence Poly(9,9-dioctylfluorene) Solvent-Tuned Aggregate Films" Polymers 14, no. 8: 1615. https://doi.org/10.3390/polym14081615