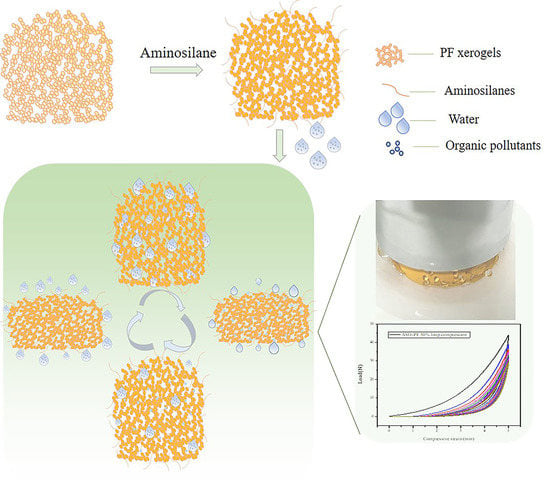
Respiratory Adsorption of Organic Pollutants in Wastewater by Superhydrophobic Phenolic Xerogels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Preparation
2.2.1. Synthesis of Methyl-Order Phenolic Resin
2.2.2. Preparation of Phenolic Xerogel (PF)
2.2.3. Amino Silane Modified Xerogel (ASO-PF)
2.3. Characterization
2.4. Oil–Water Separation Test
2.5. Adsorption of Phenol
2.6. Dye Adsorption
3. Results and Discussion
3.1. Material Synthesis and Structural Characterization
3.2. Compression Performance of PF and ASO-PF
3.3. Oil–Water Separation Performance of the PF and ASO-PF
3.4. Adsorption Capacity of ASO-PF on Phenol
3.4.1. Effect of pH Value on the Phenol Adsorption Capacity of ASO-PF
3.4.2. Adsorption Kinetics and Adsorption Isotherms of ASO-PF
3.4.3. Adsorption of Organic Dyes by ASO-PF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Kong, L.; Dai, Y.; Yue, X.; Rong, J.; Qiu, F.; Pan, J. Enhanced oils and organic solvents absorption by polyurethane foams composites modified with MnO2 nanowires. Chem. Eng. J. 2017, 309, 7–14. [Google Scholar] [CrossRef]
- Zhang, R.; Quan, S.; Xia, M.; Wang, Q.; Zhang, W.; Yang, J. Effect of surface charge status of amorphous porous coordination polymer particles on the adsorption of organic dyes from an aqueous solution. J. Colloid Interface Sci. 2018, 525, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.; Zhang, Y.; Wang, A. Mussel and fish scale-inspired underwater superoleophobic kapok membranes for continuous and simultaneous removal of insoluble oils and soluble dyes in water. J. Mater. Chem. A 2015, 3, 18475–18482. [Google Scholar] [CrossRef]
- Moshe, S.B.; Rytwo, G. Thiamine-based organoclay for phenol removal from water. Appl. Clay Sci. 2018, 155, 50–56. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Wang, D.; Liu, S.; Li, X.; Zhou, X.; Yi, H.; Li, B.; Zhang, M.; Li, L.; et al. In situ chemical oxidation: Peroxide or persulfate coupled with membrane technology for wastewater treatment. J. Mater. Chem. A 2021, 9, 11944–11960. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste Conversion and Resource Recovery from Wastewater by Ion Exchange Membranes: State-of-the-Art and Perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Tomei, M.C.; Mosca Angelucci, D.; Clagnan, E.; Brusetti, L. Anaerobic biodegradation of phenol in wastewater treatment: Achievements and limits. Appl. Microbiol. Biotechnol. 2021, 105, 2195–2224. [Google Scholar] [CrossRef]
- Yang, F.; Yang, P. Biopolymer-Based Membrane Adsorber for Removing Contaminants from Aqueous Solution: Progress and Prospects. Macromol. Rapid Commum. 2022, 43, 2100669. [Google Scholar] [CrossRef]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Yahaya, A.; Okoh, O.O.; Agunbiade, F.O.; Okoh, A.I. Occurrence of phenolic derivatives in Buffalo River of Eastern Cape South Africa: Exposure risk evaluation. Ecotoxicol. Environ. Saf. 2019, 171, 887–893. [Google Scholar] [CrossRef]
- Sultan, M. Polyurethane for removal of organic dyes from textile wastewater. Environ. Chem. Lett. 2017, 15, 347–366. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Borrull, J.; Colom, A.; Fabregas, J.; Borrull, F.; Pocurull, E. Presence, behaviour and removal of selected organic micropollutants through drinking water treatment. Chemosphere 2021, 276, 130023. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shunmugam, R. Polymer based gels and their applications in remediation of dyes from textile effluents. J. Macromol. Sci. Part A 2020, 57, 906–926. [Google Scholar] [CrossRef]
- Yagoub, H.; Zhu, L.; Shibraen, M.H.M.A.; Altam, A.A.; Babiker, D.M.D.; Liang, S.; Jin, Y.; Yang, S. Complex Aerogels Generated from Nano-Polysaccharides and Its Derivatives for Oil–Water Separation. Polymers 2019, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Haghbeen, K.; Legge, R.L. Adsorption of phenolic compounds on some hybrid xerogels. Chem. Eng. J. 2009, 150, 1–7. [Google Scholar] [CrossRef]
- Pillai, A.; Kandasubramanian, B. Carbon Xerogels for Effluent Treatment. J. Chem. Eng. Data 2020, 65, 2255–2270. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Li, P.; Liu, F.; Guo, H.; Li, W. Recent advances of organogels: From fabrications and functions to applications. Prog. Org. Coat. 2021, 159, 106417. [Google Scholar] [CrossRef]
- Ren, S.; Sun, P.; Wu, A.; Sun, N.; Sun, L.; Dong, B.; Zheng, L. Ultra-fast self-healing PVA organogels based on dynamic covalent chemistry for dye selective adsorption. N. J. Chem. 2019, 43, 7701–7707. [Google Scholar] [CrossRef]
- Hernández-Abreu, A.B.; Álvarez-Torrellas, S.; Águeda, V.I.; Larriba, M.; Delgado, J.A.; Calvo, P.A.; García, J. Enhanced removal of the endocrine disruptor compound Bisphenol A by adsorption onto green-carbon materials. Effect of real effluents on the adsorption process. J. Environ. Manag. 2020, 266, 110604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Sriram, G.; Bhat, M.P.; Kigga, M.; Uthappa, U.T.; Jung, H.; Kumeria, T.; Kurkuri, M.D. Amine activated diatom xerogel hybrid material for efficient removal of hazardous dye. Mater. Chem. Phys. 2019, 235, 121738. [Google Scholar] [CrossRef]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Synthesis of carbon xerogels modified with amine groups and copper for efficient adsorption of caffeine. Chem. Eng. J. 2018, 345, 13–21. [Google Scholar] [CrossRef]
- Yang, T.; Dong, Y.; Li, X.; Zhang, J.; Cheng, J. Pore structure of the phenolic porous material and its mechanical property. J. Cell. Plast. 2014, 51, 401–412. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Zhang, C. Shrinkage and pore structure in preparation of carbon aerogels. J. Sol-Gel Sci. Technol. 2011, 59, 371–380. [Google Scholar] [CrossRef]
- Mukherjee, S.; Yang, J.W.; Hoffmann, S.; List, B. Asymmetric Enamine Catalysis. Chem. Rev. 2007, 107, 5471–5569. [Google Scholar] [CrossRef]
- Tsunoji, N.; Ikeda, T.; Sadakane, M.; Sano, T. Synthesis and characteristics of novel layered silicate HUS-7 using benzyltrimethylammonium hydroxide and its unique and selective phenol adsorption behavior. J. Mater. Chem. A 2014, 2, 3372–3380. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Afonso, R.; Gales, L.; Mendes, A. Kinetic derivation of common isotherm equations for surface and micropore adsorption. Adsorption 2016, 22, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Asnin, L.D.; Fedorov, A.A.; Chekryshkin, Y.S. Thermodynamic quantities of adsorption described by Freundlich isotherm. Russ. Chem. Bull. 2000, 49, 178–180. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Bullen, J.C.; Saleesongsom, S.; Gallagher, K.; Weiss, D.J. A Revised Pseudo-Second-Order Kinetic Model for Adsorption, Sensitive to Changes in Adsorbate and Adsorbent Concentrations. Langmuir 2021, 37, 3189–3201. [Google Scholar] [CrossRef]
- Rahman, M.M.; Muttakin, M.; Pal, A.; Shafiullah, A.Z.; Saha, B.B. A Statistical Approach to Determine Optimal Models for IUPAC-Classified Adsorption Isotherms. Energies 2019, 12, 4565. [Google Scholar] [CrossRef] [Green Version]
- Borba, A.; Almangano, M.; Portugal, A.A.; Patrício, R.; Simões, P.N. Methylsilsesquioxane-Based Aerogel Systems—Insights into the Role of the Formation of Molecular Clusters. J. Phys. Chem. A 2016, 120, 4079–4088. [Google Scholar] [CrossRef]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Carracedo-Pérez, M.; Puertas, M.L.; Esteban-Cubillo, A.; Santaren, J.; Villafañe, F.; Rodríguez-Pérez, M. Characterization and Properties of Water-Blown Rigid Polyurethane Foams Reinforced with Silane-Modified Nanosepiolites Functionalized with Graphite. Materials 2022, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, F.; Dai, X.; Jiao, L.; Yao, H.; Du, Z.; Wang, H.; Qiu, X. Surface modification of high-performance polyimide fibres by using a silane coupling agent. Compos. Interface 2019, 26, 687–698. [Google Scholar] [CrossRef]
- Jang, M.; Park, C.K.; Lee, N.Y. Modification of polycarbonate with hydrophilic/hydrophobic coatings for the fabrication of microdevices. Sens. Actuators B Chem. 2014, 193, 599–607. [Google Scholar] [CrossRef]










| Sample | Temperature °C | Sample Mass g | Apparent Density g·cm−3 | True Density g·cm−3 | Porosity% |
|---|---|---|---|---|---|
| PF | 23.41 | 0.2872 | 0.113 | 1.4129 | 92.0 |
| ASO-PF | 23.36 | 0.2943 | 0.134 | 1.4538 | 90.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gong, D.; Zhou, Y.; Zhang, C.; Zhang, C.; Sheng, Y.; Peng, S. Respiratory Adsorption of Organic Pollutants in Wastewater by Superhydrophobic Phenolic Xerogels. Polymers 2022, 14, 1596. https://doi.org/10.3390/polym14081596
Li Y, Gong D, Zhou Y, Zhang C, Zhang C, Sheng Y, Peng S. Respiratory Adsorption of Organic Pollutants in Wastewater by Superhydrophobic Phenolic Xerogels. Polymers. 2022; 14(8):1596. https://doi.org/10.3390/polym14081596
Chicago/Turabian StyleLi, Yinchun, Depeng Gong, Youliang Zhou, Chaocan Zhang, Chunyang Zhang, Yitian Sheng, and Shu Peng. 2022. "Respiratory Adsorption of Organic Pollutants in Wastewater by Superhydrophobic Phenolic Xerogels" Polymers 14, no. 8: 1596. https://doi.org/10.3390/polym14081596