Study of a Novel Electrochromic Device with Crystalline WO3 and Gel Electrolyte
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of PADA Gel Polymer
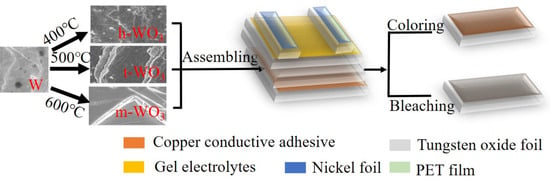
2.3. Assembly of PECD
2.4. Characterization
3. Results and Discussion
3.1. Optical Properties of Electrochromic Devices
3.2. Cyclic Stability of Electrochromic Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Li, H.Z.; Yu, W.W.; Elezzabi, A.Y. Transparent inorganic multicolour displays enabled by zinc-based electrochromic devices. Light Sci. Appl. 2020, 9, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.X.; Zhang, Y.J.; Ma, L.; Zhao, S.; Wu, N.; Xiao, D. Easy-to-make sulfonatoalkyl viologen/sodium carboxymethylcellulose hydrogel-based electrochromic devices with high coloration efficiency, fast response and excellent cycling stability. Dye. Pigment 2020, 174, 108055. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Ma, J.M.; Cao, Y.L.; Deng, Y.H. Electrochromic Metal Oxides: Recent Progress and Prospect. Adv. Electron. Mater. 2018, 4, 1800185–1800204. [Google Scholar] [CrossRef]
- Yang, P.H.; Sun, P.; Mai, W.J. Electrochromic energy storage devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for smart windows: Oxide-based thin films and devices. Thin Solid Film. 2014, 564, 1–38. [Google Scholar] [CrossRef]
- Granqvist, C.G. Oxide electrochromics: An introduction to devices and materials. Sol. Energy Mater. Sol. Cells 2012, 99, 1–13. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Yan, L.; Meng, H.; He, Y.; Yao, C.; Xu, P.; Zhang, X.; Hu, W.; Huang, W. Side-chain engineering of green color electrochromic polymer materials: Toward adaptive camouflage application. J. Mater. Chem. C 2016, 4, 2269–2273. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.L.; Wang, Y.; Zou, X.L.; Tan, Y.; Jia, C.Y.; Weng, X.L.; Deng, L.G. Infrared electrochromic materials, devices and applications. Appl. Mater. Today 2021, 24, 101073. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.Y.; Cong, S.; Chen, J.; Sun, H.Z.; Chen, Z.G.; Song, G.; Geng, F.X.; Chen, Q.; Zhao, Z.G. Towards full-colour tunability of inorganic electrochromic devices using ultracompact fabry-perot nanocavities. Nat. Commun. 2020, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Shenouda, S.S.; AlAbdulaa, T.H.; Zahran, H.Y.; Yahia, I.S. Synthesis, structure identification and linear/nonlinear optics of hydrothermally grown WO3 nanostructured thin film/FTO: Novel approach. Ceram. Int. 2021, 11, 311. [Google Scholar] [CrossRef]
- Lakshmi, M.; Avani, A.V.; Kathirvel, P.; Marnadu, R.; Packiaraj, R.; Joshua, J.R.; Nallamuthu, N.; Shkir, M.; Saravanakumar, S. Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications. J. Alloys Compd. 2021, 882, 160670. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Liu, Q.Y.; Hong, R.J.; Tao, C.X.; Wang, Q.; Lin, H.; Han, Z.X.; Zhang, D.W. SERS-active WO3-x thin films with tunable surface plasmon resonance induced by defects from thermal treatment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 268, 120686. [Google Scholar] [CrossRef]
- Muñoz-Bolaños, J.D.; Rodríguez-Páez, J.E. WO3 mono-nanocrystals: Synthesis, characterization and evaluation of their electrical behavior in oxygen and acetone atmospheres. Mater. Sci. Eng. B 2021, 274, 115472. [Google Scholar] [CrossRef]
- Oscar, H.-A.; Rafael, A.; Aníbal, S. DFT+U study of the electronic structure changes of WO3 monoclinic and hexagonal surfaces upon Cu, Ag, and Au adsorption. Applications for CO adsorption. Surf. Sci. 2021, 714, 121907. [Google Scholar] [CrossRef]
- Lyu, H.L. Triple layer tungsten trioxide, graphene, and polyaniline composite films for combined energy storage and electrochromic applications. Polymers 2019, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Green, S.; Niklasson, G.A.; Mlyuka, N.R.; von Kræmer, S.; Georén, P. Advances in chromogenic materials and devices. Thin Solid Film. 2010, 518, 3046–3053. [Google Scholar] [CrossRef]
- Perfecto, T.M.; Zito, C.A.; Volanti, D.P. Design of nanostructured WO3·0.33 H2O via combination of ultrasonic spray nozzle and microwave-assisted hydrothermal methods for enhancing isopropanol gas sensing at room temperature. CrystEngComm 2017, 19, 2733–2738. [Google Scholar] [CrossRef]
- Chang, X.T.; Hu, R.R.; Sun, S.B.; Liu, J.R.; Lei, Y.H.; Liu, T.; Dong, L.H.; Yin, Y.S. Sunlight-charged electrochromic battery based on hybrid film of tungsten oxide and polyaniline. Appl. Surf. Sci. 2018, 441, 105–112. [Google Scholar] [CrossRef]
- Ahmadian, H.; Tehrani, F.S.; Aliannezhadi, M. Hydrothermal synthesis and characterization of WO3 nanostructures: Effects of capping agent and pH. Mater. Res. Express 2019, 6, 105024. [Google Scholar] [CrossRef]
- Abbaspoor, M.; Aliannezhadi, M.; Tehrani, F.S. Effect of solution pH on as-synthesized and calcined WO3 nanoparticles synthesized using sol-gel method. Opt. Mater. 2021, 121, 111552. [Google Scholar] [CrossRef]
- Pooyodying, P.; Son, Y.-H.; Sung, Y.-M.; Ok, J.-W. The effect of sputtering Ar gas pressure on optical and electrical properties of flexible ECD device with WO3 electrode deposited by RF magnetron sputtering on ITO/PET substrate. Opt. Mater. 2022, 123, 111829. [Google Scholar] [CrossRef]
- Sharma, A.; Saini, A.K.; Kumar, N.; Tejwan, N.; Singh, T.A.; Thakur, V.K.; Das, J. Methods of preparation of metal-doped and hybrid tungsten oxide nanoparticles for anticancer, antibacterial, and biosensing applications. Surf. Interfaces 2022, 28, 101641. [Google Scholar] [CrossRef]
- Wei, S.H.; Zhao, J.H.; Hu, B.X.; Wu, K.Q.; Du, W.M.; Zhou, M.H. Hydrothermal synthesis and gas sensing properties of hexagonal and orthorhombic WO3 nanostructures. Ceram. Int. 2017, 43, 2579–2585. [Google Scholar] [CrossRef]
- Han, X.P.; Amrane, N.; Qamhieh, N.; Benkraouda, M. Enhanced optoelectronic functionality of N + H codoped monoclinic WO3: A hybrid functional study. Chem. Phys. 2021, 549, 111283. [Google Scholar] [CrossRef]
- Hajiahmadi, Z.; Azar, Y.T. Computational study of h-WO3 surfaces as a semiconductor in water-splitting application. Surf. Interfaces 2021, 28, 101695. [Google Scholar] [CrossRef]
- Feng, M.C.; Liu, Y.N.; Zhao, Z.Y.; Huang, H.W.; Peng, Z.J. The preparation of Fe doped triclinic-hexagonal phase heterojunction WO3 film and its enhanced photocatalytic reduction of Cr (VI). Mater. Res. Bull. 2019, 109, 168–174. [Google Scholar] [CrossRef]
- Jamali, M.; Tehrani, F.S. Effect of synthesis route on the structural and morphological properties of WO3 nanostructures. Mater. Sci. Semicond. Process. 2020, 107, 104829. [Google Scholar] [CrossRef]
- Jamali, M.; Tehrani, F.S. Thermally stable WO3 nanostructure synthesized by hydrothermal method without using surfactant. Mater. Sci. Eng. B 2021, 270, 115221. [Google Scholar] [CrossRef]
- Jayatissa, A.H.; Dadi, A.; Aoki, T. Nanocrystalline WO3 films prepared by two-step annealing. Appl. Surf. Sci. 2005, 244, 453–457. [Google Scholar] [CrossRef]
- Li, T.Y.; Li, X.L.; Bae, J.Y.; Kim, S.H.; Moon, H.C. Non-volatile, Li-doped ion gel electrolytes for flexible WO3-based electrochromic devices. Mater. Des. 2019, 162, 45–51. [Google Scholar] [CrossRef]
- Chen, W.Y.; Zhu, C.Z.; Guo, L.; Yan, M.Y.; Wu, L.L.; Zhu, B.; Qi, C.J.; Liu, S.Y.; Zhang, H.; Peng, Y. A novel ionically crosslinked gel polymer electrolyte as iontransport layer for high performance electrochromic devices. J. Mater. Chem. C 2019, 7, 3744–3750. [Google Scholar] [CrossRef]
- Chen, P.; Liang, X.P.; Wang, J.; Zhang, D.; Yang, S.M.; Wu, W.S.; Zhang, W.; Fan, X.W.; Zhang, D.Q. PEO/PVDF-based gel polymer electrolyte by incorporating nano-TiO2 for electrochromic glass. J. Sol-Gel Sci. Technol. 2016, 81, 850–858. [Google Scholar] [CrossRef]
- Li, F.-W.; Yen, T.-C.; Liou, G.-S. Synthesis of high-performance electrochromic material for facile fabrication of truly black electrochromic devices. Electrochim. Acta 2021, 367, 137474. [Google Scholar] [CrossRef]
- Ramadan, R.; Kamal, H.; Hashem, H.M.; Abdel, H.K. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Sol. Energy Mater. Sol. Cells 2014, 127, 147–156. [Google Scholar] [CrossRef]
- Dulgerbaki, C.; Oksuz, A.U. Poly (3-methylthiophene)/Tungsten oxide hybrid materials for highly efficient electrochromic devices. Opt. Mater. 2021, 119, 111354. [Google Scholar] [CrossRef]
- Xiang, S.F.; Chen, S.S.; Yao, M.T.; Zheng, F.; Lu, Q.H. Strain sensor based on a flexible polyimide ionogel for application in high-and low-temperature environments. J. Mater. Chem. C 2019, 7, 9625–9632. [Google Scholar] [CrossRef]
- Hsiao, L.-Y.; Chang, T.-H.; Lu, H.-C.; Wang, Y.-C.; Lu, Y.-A.; Ho, K.-C.; Higuchi, M. A panchromatic electrochromic device composed of Ru (II)/Fe (II)-based heterometallo-supramolecular polymer. J. Mater. Chem. C 2019, 7, 7554–7562. [Google Scholar] [CrossRef]
- Kumar, G.G.; Munichandraiah, N. A gel polymer electrolyte of magnesium triflate. Solid State Ion. 2000, 128, 203–210. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Chen, W.; Liu, S.; Guo, L.; Zhang, G.; Zhang, H.; Cao, M.; Wu, L.; Xiang, T.; Peng, Y. A Self-Healing Ionic Liquid-Based Ionically Cross-Linked Gel Polymer Electrolyte for Electrochromic Devices. Polymers 2021, 13, 742. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.B.; Meng, X.H.; Liu, A.M.; Chen, Y.; Ma, T.L. A cross-linked tin oxide/polymer composite gel electrolyte with adjustable porosity for enhanced sodium ion batteries. Chem. Eng. J. 2021, 431, 133922. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.T.; Li, G.Q.; Wang, C.; Li, B.; Li, M.; Wang, Y.; Ming, H.; Wen, Y.H.; Qiu, J.Y.; et al. A cross-linked gel polymer electrolyte employing cellulose acetate matrix and layered boron nitride filler prepared via in situ thermal polymerization. J. Power Sources 2021, 484, 229235. [Google Scholar] [CrossRef]
- Petruleviciene, M.; Juodkazyte, J.; Parvin, M.; Tereshchenko, A.; Ramanavicius, S.; Karpicz, R.; Samukaite-Bubniene, U.; Ramanavicius, A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials 2020, 13, 2814. [Google Scholar] [CrossRef]
- Ram, J.; Singh, R.G.; Gupta, R.; Kumar, V.; Singh, F.; Kumar, R. Effect of annealing on the surface morphology, optical and and structural properties of nanodimensional tungsten oxide prepared by coprecipitation technique. J. Electron. Mater. 2019, 48, 1174–1183. [Google Scholar] [CrossRef]
- Shendage, S.S.; Patil, V.L.; Vanalakar, S.A.; Harale, N.S.; Bhosale, J.L.; Kim, J.H.; Payil, P.S. Sensitive and selective NO2 gas sensor based on WO3 nanoplates. Sens. Actuators B Chem. 2017, 240, 426–433. [Google Scholar] [CrossRef]
- Balaji, S.; Djaoued, Y.; Albert, A.S.; Brüning, R.; Beaudoin, N.; Robichaud, J. Porous orthorhombic tungsten oxide thin films: Synthesis, characterization, and application in electrochromic and photochromic devices. J. Mater. Chem. 2011, 21, 3940–3948. [Google Scholar] [CrossRef]
- Afify, H.H.; Hassan, S.A.; Obaida, M.; Moussa, I.; Abouelsayed, A. Preparation, characterization, and optical spectroscopic studies of nanocrystalline tungsten oxide WO3. Opt. Laser Technol. 2019, 111, 604–611. [Google Scholar] [CrossRef]










| PADA-a | PADA-b | PADA-c | |
|---|---|---|---|
| PC (mL) | 2.5 | 2.5 | 2.5 |
| DMF (mL) | 2.5 | 2.5 | 2.5 |
| LiClO4 (g) | 0.5032 | 0.5032 | 0.5032 |
| DEAM (mL) | 0.2 | 0.5 | 0.8 |
| AA (mL) | 0.8 | 0.5 | 0.2 |
| AAm (mg) | 250 | 250 | 250 |
| AIBN (mg) | 1.25 | 1.25 | 1.25 |
| Element | Tungsten | WO3-400 | WO3-500 | WO3-600 | |
|---|---|---|---|---|---|
| Weight% | O | 0 | 16.69 | 21.21 | 31.32 |
| W | 100 | 83.31 | 78.79 | 68.68 | |
| Atomic% | O | 0 | 69.71 | 75.57 | 83.98 |
| W | 100 | 30.29 | 24.43 | 16.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, G.; Wu, L.; Liu, S.; Cao, M.; Yang, Y.; Peng, Y. Study of a Novel Electrochromic Device with Crystalline WO3 and Gel Electrolyte. Polymers 2022, 14, 1430. https://doi.org/10.3390/polym14071430
Chen W, Zhang G, Wu L, Liu S, Cao M, Yang Y, Peng Y. Study of a Novel Electrochromic Device with Crystalline WO3 and Gel Electrolyte. Polymers. 2022; 14(7):1430. https://doi.org/10.3390/polym14071430
Chicago/Turabian StyleChen, Wanyu, Guixia Zhang, Lili Wu, Siyuan Liu, Meng Cao, Ying Yang, and Yong Peng. 2022. "Study of a Novel Electrochromic Device with Crystalline WO3 and Gel Electrolyte" Polymers 14, no. 7: 1430. https://doi.org/10.3390/polym14071430