Structure and Properties of Cellulose/Mycelium Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microfibrillar Cellulose (CMF) Production
2.2.2. Biotransformation of Microfibrillar Cellulose (CMF) and Nanofibrillar Cellulose (CNF)
2.2.3. Elemental Analysis
2.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Evaluation of the Biotransformation Degree
2.2.7. Laser Diffraction
2.2.8. Thermogravimetry Analysis (TGA)
3. Results and Discussion
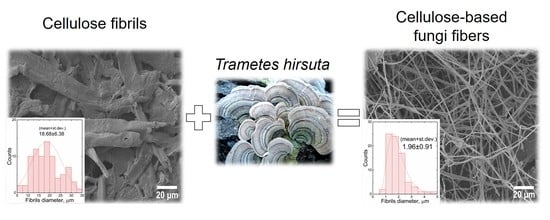
3.1. Biotransformation of Fibrillar Cellulose Using Trametes hirsuta
3.2. Biotransformation Dynamics of Fibrillar Cellulose
3.3. Laser Diffraction
3.4. The Composition Characterization
3.5. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant Extract-Loaded Bacterial Cellulose Composite Membrane for Potential Biomedical Applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Abidi, N.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers 2021, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.G.; Santos, D.F.; Ferreira, R.R.; Pinto, V.Z.; Rosa, D.S. Innovative Process for Obtaining Modified Nanocellulose from Soybean Straw. Int. J. Biol. Macromol. 2020, 165, 1803–1812. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A Review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Bauli, C.R.; Rocha, D.B.; de Oliveira, S.A.; Rosa, D.S. Cellulose Nanostructures from Wood Waste with Low Input Consumption. J. Clean. Prod. 2019, 211, 199–216. [Google Scholar] [CrossRef]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Tingaut, P.; Maniura-Weber, K.; Zimmermann, T.; Thöny-Meyer, L.; Faccio, G.; Ihssen, J. TEMPO-Oxidized Nanofibrillated Cellulose as a High Density Carrier for Bioactive Molecules. Biomacromolecules 2015, 16, 3640–3650. [Google Scholar] [CrossRef]
- Puangsin, B.; Soeta, H.; Saito, T.; Isogai, A. Characterization of Cellulose Nanofibrils Prepared by Direct TEMPO-Mediated Oxidation of Hemp Bast. Cellulose 2017, 24, 3767–3775. [Google Scholar] [CrossRef]
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous Preparation of Cellulose Nanocrystals and Micron-Sized Porous Colloidal Particles of Cellulose by TEMPO-Mediated Oxidation. Green Chem. 2015, 17, 808–811. [Google Scholar] [CrossRef]
- Chen, X.Q.; Deng, X.Y.; Shen, W.H.; Jia, M.Y. Preparation and Characterization of the Spherical Nanosized Cellulose by the Enzymatic Hydrolysis of Pulp Fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose Production: Exploring the Enzymatic Route and Residues of Pulp and Paper Industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and Mechanics of Fungal Mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 374. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Antinori, M.E.; Contardi, M.; Suarato, G.; Armirotti, A.; Bertorelli, R.; Mancini, G.; Debellis, D.; Athanassiou, A. Advanced Mycelium Materials as Potential Self-Growing Biomedical Scaffolds. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef]
- Zimele, Z.; Irbe, I.; Grinins, J.; Bikovens, O.; Verovkins, A.; Bajare, D. Novel Mycelium-Based Biocomposites (MBB) as Building Materials. J. Renew. Mater. 2020, 8, 1067–1076. [Google Scholar] [CrossRef]
- Antinori, M.E.; Ceseracciu, L.; Mancini, G.; Heredia-Guerrero, J.A.; Athanassiou, A. Fine-Tuning of Physicochemical Properties and Growth Dynamics of Mycelium-Based Materials. ACS Appl. Bio Mater. 2020, 3, 1044–1051. [Google Scholar] [CrossRef]
- Filipova, I.; Irbe, I.; Spade, M.; Skute, M.; Dāboliņa, I.; Baltiņa, I.; Vecbiskena, L. Mechanical and Air Permeability Performance of Novel Biobased Materials from Fungal Hyphae and Cellulose Fibers. Materials 2021, 14, 136. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully Bio-Based Hybrid Composites Made of Wood, Fungal Mycelium and Cellulose Nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef]
- Attias, N.; Reid, M.; Mijowska, S.C.; Dobryden, I.; Isaksson, M.; Pokroy, B.; Grobman, Y.J.; Abitbol, T. Biofabrication of Nanocellulose–Mycelium Hybrid Materials. Adv. Sustain. Syst. 2021, 5, 2000196. [Google Scholar] [CrossRef]
- Kozhevnikova, E.Y.; Barkov, A.V.; Spitsyna, E.A.; Petrova, D.A.; Novikov, A.A.; Kotelev, M.S.; Gushchin, P.A.; Ivanov, E.V.; Vinokurov, V.A. Basidiomycete Strain Trametes Hirsuta. RU 2614263 C1, 24 March 2017. [Google Scholar]
- Coughlan, M.P. Cellulose Degradation by Fungi. In Microbial Enzymes and Biotechnology; Fogarty, W.M., Kelly, C.T., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 1–36. [Google Scholar] [CrossRef]
- Baldrian, P.; Valášková, V. Degradation of Cellulose by Basidiomycetous Fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [Green Version]
- Freitas, G.A.D.; Almeida, A.F.D.; Conceição, R.C.N.D.; Luz, J.H.S.D.; Nunes, B.H.D.N.; Ribeiro, E.A.; Ribeiro, F.D.S.; Neto, A.A.; Machado, Â.F.; Deusdará, T.T.; et al. Fungi with Cellulolytic Potential: Screening, Inoculum, and Methodology for Isolation. Int. J. Adv. Eng. Res. Sci. 2019, 6, 520–527. [Google Scholar] [CrossRef]
- Várnai, A.; Mäkelä, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-Binding Modules of Fungal Cellulases. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar]
- Angelova, G.V.; Brazkova, M.S.; Krastanov, A.I. Renewable Mycelium Based Composite—Sustainable Approach for Lignocellulose Waste Recovery and Alternative to Synthetic Materials—A Review. Z. Naturforsch. Sect. C J. Biosci. 2021, 76, 431–442. [Google Scholar] [CrossRef]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and Characterisation of Cellulose Nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.; Li, K.; Jung, D.; Park, K.; Lee, J. Preparation and Characterization of Various Chitin-Glucan Complexes Derived from White Button Mushroom Using a Deep Eutectic Solvent-Based Ecofriendly Method. Int. J. Biol. Macromol. 2021, 169, 122–129. [Google Scholar] [CrossRef]
- Smirnou, D.; Krcmar, M.; Prochazkova, E. Chitin-Glucan Complex Production by Schizophyllum Commune Submerged Cultivation. Pol. J. Microbiol. 2011, 60, 223–228. [Google Scholar] [CrossRef]
- Roca, C.; Chagas, B.; Farinha, I.; Freitas, F.; Mafra, L.; Aguiar, F.; Oliveira, R.; Reis, M.A.M. Production of Yeast Chitin–Glucan Complex from Biodiesel Industry Byproduct. Process Biochem. 2012, 47, 1670–1675. [Google Scholar] [CrossRef]
- Hong, Y.; Ying, T. Characterization of a Chitin-Glucan Complex from the Fruiting Body of Termitomyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 1, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorizat. 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Araújo, D.; Voisin, P.; Alves, V.D.; Rosatella, A.A.; Afonso, C.A.M.; Freitas, F.; Neves, L.A. Chitin-Glucan Complex—Based Biopolymeric Structures Using Biocompatible Ionic Liquids. Carbohydr. Polym. 2020, 247, 116679. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Hrdina, R.; Abdel-Mohsen, A.M.; Fouda, M.M.G.; Soliman, A.Y.; Mohamed, F.K.; Mohsin, K.; Pinto, T.D. Chitin and Chitosan from Brazilian Atlantic Coast: Isolation, Characterization and Antibacterial Activity. Int. J. Biol. Macromol. 2015, 80, 107–120. [Google Scholar] [CrossRef]
- Araújo, D.; Alves, V.D.; Marques, A.C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Low Temperature Dissolution of Yeast Chitin-Glucan Complex and Characterization of the Regenerated Polymer. Bioengineering 2020, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- César, E.; Canche-Escamilla, G.; Montoya, L.; Ramos, A.; Duarte-Aranda, S.; Bandala, V.M. Characterization and Physical Properties of Mycelium Films Obtained from Wild Fungi: Natural Materials for Potential Biotechnological Applications. J. Polym. Environ. 2021, 29, 4098–4105. [Google Scholar] [CrossRef]





| Biopolymer | C, % | H, % | N, % |
|---|---|---|---|
| CMF | 44.03 ± 0.06 | 6.42 ± 0.07 | 0 |
| CMFmodified | 43.78 ± 0.12 | 6.29 ± 0.01 | 1 ± 0.10 |
| Chitin (Crustaceans) | 37.3 ± 0.08 | 6.09 ± 0.07 | 5.5 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayfutdinova, A.; Samofalova, I.; Barkov, A.; Cherednichenko, K.; Rimashevskiy, D.; Vinokurov, V. Structure and Properties of Cellulose/Mycelium Biocomposites. Polymers 2022, 14, 1519. https://doi.org/10.3390/polym14081519
Sayfutdinova A, Samofalova I, Barkov A, Cherednichenko K, Rimashevskiy D, Vinokurov V. Structure and Properties of Cellulose/Mycelium Biocomposites. Polymers. 2022; 14(8):1519. https://doi.org/10.3390/polym14081519
Chicago/Turabian StyleSayfutdinova, Adeliya, Irina Samofalova, Artem Barkov, Kirill Cherednichenko, Denis Rimashevskiy, and Vladimir Vinokurov. 2022. "Structure and Properties of Cellulose/Mycelium Biocomposites" Polymers 14, no. 8: 1519. https://doi.org/10.3390/polym14081519