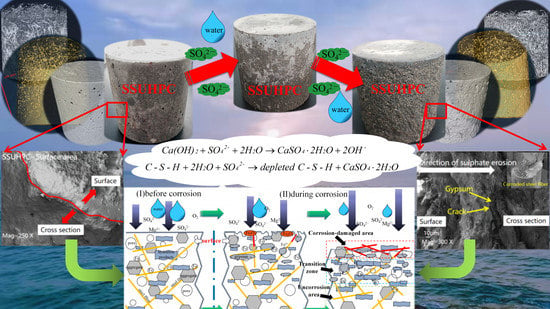
Sulphate Corrosion Mechanism of Ultra-High-Performance Concrete (UHPC) Prepared with Seawater and Sea Sand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mixtures, Specimen Preparation and Exposure Conditions
2.3. Test Methods
2.3.1. Sulphate Corrosion Test
2.3.2. Compressive Strength and Flexural Strength
2.3.3. Vickers Hardness
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. X-ray Diffraction (XRD)
2.3.6. Mercury Intrusion Porosimetry (MIP)
2.3.7. X-CT Test and Data Analysis
3. Results and Discussion
3.1. Compressive Strength and Flexural strength
3.2. Change of appearance
3.3. Internal Characteristics
3.3.1. Variation of Cement Mortar
3.3.2. Pore and Fiber Structure Analysis Based on 3D Modeling
3.4. Microstructure Characterization
3.5. Discussion
4. Conclusions
- The evolution of UHPC under sulphate corrosion: (a) The cement stone in contact with sulphate was dissolved under the action of erosion, and the aggregate on the surface of the concrete was exposed and became rough. (b) As erosion developed, mortar on the surface of the concrete lost its cementation and gradually flaked off. Iron ions in steel fibers on the surface of UHPC were precipitated, and rust was formed on the surface. (c) With further sulphate erosion, the peeling degree of mortar on the surface was further aggravated, and the steel fibers on the surface were exposed and corroded. (d) With the continuous action of sulphate corrosion, the severely corroded steel fibers and weakened mortar with reduced cementation force on the surface of the concrete fell off. New mortar and unrusted steel fibers were exposed, and the process of (b)~(d) was repeated.
- Compared with SSUHPC, FRUHPC was damaged more seriously by sulphate erosion. Vickers hardness results showed that the strength on the surface area (from 0 to 1 mm) of SSUHPC decreased, whereas the surface area of FRUHPC from 0 to 8 mm suffered a more serious loss of strength. Within a zone between the outer surface and the interior of SSUHPC and FRUHPC, the Vickers hardness of mortar was increased by 5~15%. Ultra-high-performance concrete made from seawater and sea sand was found to have better sulphate resistance than that made with fresh water and river sand.
- X-CT test results showed that the structure of SSUHPC was relatively uniform, whereas the gray values of the outer surface and the inner surface in FRUHPC were obviously different. The concrete structure did not appear to exhibit volume expansion or cracking, and the pore structure and steel fiber structure did not show obvious changes. Although the surface of the concrete showed obvious spalling of mortar and steel fiber after sulphate corrosion, there was no obvious abnormality in the interior. Both SSUHPC and FRUHPC showed excellent sulphate resistance.
- The surface area of UHPC was damaged, and spalling occurred with a 1~2 mm thickness due to sulphate corrosion. A large number of gypsum, Friedel’s salt and rehydration products appeared on the surface (within 1 mm) of SSUHPC and FRUHPC. Steel fibers were corroded and exposed on the surface of the concrete, presenting serious sulphate corrosion characteristics. There was no obvious structural damage at a depth of 1–5 mm, but a high content of sulphate corrosion products, such as gypsum, was present, and the porosity of SSUHPC was obviously reduced by 36.99%. However, there was no obvious abnormality inside the concrete, and the rehydration in this area was obvious.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santhanam, M.; Cohen, M.D.; Olek, J. Modeling the effects of solution temperature and concentration during sulfate attack on cement mortars. Cem. Concr. Res. 2002, 32, 585–592. [Google Scholar] [CrossRef]
- Hor, Y.; Teo, W.; Kazutaka, S. Experimental investigation on the behaviour of reinforced concrete slabs strengthened with ultra-high performance concrete. Constr. Build. Mater. 2017, 155, 463–474. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M.; Megawati, O. Inclusion of nano metakaolin as additive in ultra high performance concrete (UHPC). Constr. Build. Mater. 2016, 127, 167–175. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, W.; Song, J.; Lee, G.C. Application of Ultra-High Performance Concrete in bridge engineering. Constr. Build. Mater. 2018, 186, 1256–1267. [Google Scholar] [CrossRef]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- Hime, W.G.; Mather, B. “Sulfate attack,” or is it? Cem. Concr. Res. 1999, 29, 789–791. [Google Scholar] [CrossRef]
- Chen, J.-k.; Jiang, M.-q. Long-term evolution of delayed ettringite and gypsum in Portland cement mortars under sulfate erosion. Constr. Build. Mater. 2009, 23, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Cwirzen, A.; Sztermen, P.; Habermehl-Cwirzen, K. Effect of Baltic Seawater and Binder Type on Frost Durability of Concrete. J. Mater. Civ. Eng. 2014, 26, 283–287. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Irassar, E.F. Ettringite formation in low C(3)A Portland cement exposed to sodium sulfate solution. Cem. Concr. Res. 1997, 27, 1061–1071. [Google Scholar] [CrossRef]
- Hagelia, P.; Sibbick, R.G. Thaumasite Sulfate Attack, Popcorn Calcite Deposition and acid attack in concrete stored at the “Blindtarmen” test site Oslo, from 1952 to 1982. Mater. Charact. 2009, 60, 686–699. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Gonzalez, M. Microstructural study of sulfate attack on ordinary and limestone Portland cements at ambient temperature. Cem. Concr. Res. 2003, 33, 31–41. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Kurtis, K.E. Time to failure for concrete exposed to severe sulfate attack. Cem. Concr. Res. 2003, 33, 987–993. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Sibbick, T.; Fenn, D.; Crammond, N. The occurrence of thaumasite as a product of seawater attack. Cem. Concr. Compos. 2003, 25, 1059–1066. [Google Scholar] [CrossRef]
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on portland cement paste I. microstructure analysis. Cem. Concr. Res. 1992, 1, 169–180. [Google Scholar] [CrossRef]
- Han, S.; Zhong, J.; Yu, Q.; Yan, L.; Ou, J. Sulfate resistance of eco-friendly and sulfate-resistant concrete using seawater sea-sand and high-ferrite Portland cement. Constr. Build. Mater. 2021, 305, 124753. [Google Scholar] [CrossRef]
- Huang, X.; Hu, S.; Wang, F.; Yang, L.; Rao, M.; Tao, Y. Enhanced Sulfate Resistance: The Importance of Iron in Aluminate Hydrates. ACS Sustain. Chem. Eng. 2019, 7, 6792–6801. [Google Scholar] [CrossRef]
- Lee, S.T.; Moon, H.Y.; Swamy, R.N. Sulfate attack and role of silica fume in resisting strength loss. Cem. Concr. Compos. 2005, 27, 65–76. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Gupta, S.; Muthukrishnan, S.; Kua, H.W. Comparing influence of inert biochar and silica rich biochar on cement mortar—Hydration kinetics and durability under chloride and sulfate environment. Constr. Build. Mater. 2021, 268, 121142. [Google Scholar] [CrossRef]
- Liu, H.; Huang, F. Experimental study on accelerated speed of concrete sulfate attack. In Proceedings of the International Conference on Civil, Architectural and Hydraulic Engineering (ICCAHE 2012), Zhangjiajie, China, 10–12 August 2012. [Google Scholar]
- Qin, L.; Gao, X.; Su, A.; Li, Q. Effect of carbonation curing on sulfate resistance of cement-coal gangue paste. J. Clean. Prod. 2021, 278, 123897. [Google Scholar] [CrossRef]
- Turchin, V.; Yudina, L.; Sattarova, A. Research Sulfate Resistance of Cement-Containing Composition. In Proceedings of the 11th International Scientific Conference on Modern Building Materials, Structures and Techniques (MBMST), Vilnius, Lithuania, 16–17 May 2013. [Google Scholar]
- Ding, Q.; Liu, Y.; Liu, X.; Geng, C.; Hu, S.; Chen, B. Effects of Sulfate Attack on Microstructure of UHPC Hydration Products under Different Curing Regimes. Bull. Chin. Ceram. Soc. 2018, 37, 772–780. [Google Scholar]
- Song, S.M.; Wei, C.X. Durability of the reactive powder concrete (RPC). Concrete 2006, 2, 72–73. (In Chinese) [Google Scholar]
- Limeira, J.; Etxeberria, M.; Agullo, L.; Molina, D. Mechanical and durability properties of concrete made with dredged marine sand. Constr. Build. Mater. 2011, 25, 4165–4174. [Google Scholar] [CrossRef]
- Liu, W.; Cui, H.; Dong, Z.; Xing, F.; Zhang, H.; Lo, T.Y. Carbonation of concrete made with dredged marine sand and its effect on chloride binding. Constr. Build. Mater. 2016, 120, 1–9. [Google Scholar] [CrossRef]
- Vafaei, D.; Hassanli, R.; Ma, X.; Duan, J.; Yan, Z. Sorptivity and mechanical properties of fiber-reinforced concrete made with seawater and dredged sea-sand. Constr. Build. Mater. 2021, 270, 121436. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.J.; Dong, B.Q. Study of the resistance to sulfate attach of concrete made with dredged marine sand. Ind. Constr. 2014, 44, 131–135. (In Chinese) [Google Scholar] [CrossRef]
- Su, Q.; Chen, A.R. Research on corrosion to washed sea sand concrete attacked by sulfate. Concrete 2013, 1, 21–23. (In Chinese) [Google Scholar]
- Zhang, Z.; Sang, Z.; Zhang, L.; Ma, Z.; Zhang, Y. Experimental Research on Durability of Concrete Made by Seawater and Sea-Sand. In Proceedings of the 2nd International Conference on Biotechnology, Chemical and Materials Engineering (CBCME 2012), Xiamen, China, 28–29 December 2013. [Google Scholar]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Joo, M.S. Mechanical and durability performance of marine sand and seawater concrete incorporating silicomanganese slag as coarse aggregate. Constr. Build. Mater. 2020, 254, 119195. [Google Scholar] [CrossRef]
- Bossa, N.; Chaurand, P.; Vicente, J.; Borschneck, D.; Levard, C.; Aguerre-Chariol, O.; Rose, J. Micro- and nano-X-ray computed-tomography: A step forward in the characterization of the pore network of a leached cement paste. Cem. Concr. Res. 2015, 67, 138–147. [Google Scholar] [CrossRef]
- Gallucci, E.; Scrivener, K.; Groso, A.; Stampanoni, M.; Margaritondo, G. 3D experimental investigation of the microstructure of cement pastes using synchrotron X-ray microtomography (μCT). Cem. Concr. Res. 2006, 37, 360–368. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Sugiyama, T.; Hitomi, T.; Takeda, N. Quantification of tortuosity in hardened cement pastes using synchrotron-based X-ray computed microtomography. Cem. Concr. Res. 2009, 39, 548–557. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Promentilla, M.A.B.; Hitomi, T.; Takeda, N. Application of synchrotron microtomography for pore structure characterization of deteriorated cementitious materials due to leaching. Cem. Concr. Res. 2009, 40, 1265–1270. [Google Scholar] [CrossRef]
- Wang, J.; Dewanckele, J.; Cnudde, V.; Vlierberghe, S.V.; Verstraete, W.; Belie, N.D. X-ray computed tomography proof of bacterial-based self-healing in concrete. Cem. Concr. Compos. 2014, 53, 289–304. [Google Scholar] [CrossRef]
- Wang, R.; Gao, X.; Zhang, J.; Han, G. Spatial distribution of steel fibers and air bubbles in UHPC cylinder determined by X-ray CT method. Constr. Build. Mater. 2018, 160, 39–47. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Cao, K.; Yu, D.; Liu, W.; Zhang, X.; Xing, F.; Zhao, D. Monitoring of steel corrosion and cracking in cement paste exposed to combined sulfate-chloride attack with X-ray microtomography. Constr. Build. Mater. 2021, 302, 124345. [Google Scholar] [CrossRef]
- Tian, W.; Han, N. Experiment Analysis of Concrete’s Mechanical Property Deterioration Suffered Sulfate Attack and Drying-Wetting Cycles. Adv. Mater. Sci. Eng. 2017, 2017, 5673985. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; She, W.; Liu, N.; Liu, Z. In situ observing the erosion process of cement pastes exposed to different sulfate solutions with X-ray computed tomography. Constr. Build. Mater. 2018, 176, 556–565. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; She, W.; Wu, Z.; Liu, Z.; Ding, Y. Nondestructive monitoring the deterioration process of cement paste exposed to sodium by X-ray computed tomography. Constr. Build. Mater. 2018, 186, 182–190. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Zuo, X.B. Effect of mineral admixtures on sulfate attack resistance of cement-based materials. J. Chin. Ceram. Soc. 2012, 40, 1119–1126. (In Chinese) [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Tan, Z.; Zhang, B. Investigating the failure process of concrete under the coupled actions between sulfate attack and drying-wetting cycles by using X-ray CT. Constr. Build. Mater. 2016, 108, 129–138. [Google Scholar] [CrossRef]
- Tian, W.; Wang, Z.; Han, N. Study of meso-damage mechanism of concrete under sulfate attack. J. Disaster Prev. Mitig. Eng. 2019, 39, 16–22. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, B.; Wang, J.; Zhang, Y.; Duan, W. Damage evolution of cement mortar with high volume slag exposed to sulfate attack. Constr. Build. Mater. 2020, 247, 118626. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zhang, W.; She, W. Study on sulfate resistance of concrete with initial damage under drying-wetting cycles. J. Sustain. Cem.-Based Mater. 2018, 7, 311–322. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 95, 368–377. [Google Scholar] [CrossRef]















| Setting Time/min | Compressive Strength/MPa | Flexural Strength/MPa | Fineness/% | Density/(g/cm3) | Water Consumption for Standard Consistency/% | |||
|---|---|---|---|---|---|---|---|---|
| Initial Setting | Final Setting | 3 d | 28 d | 3 d | 28 d | |||
| 178 | 219 | 23.1 | 51.2 | 5.4 | 9.3 | 0.8 | 3.5 | 29.5 |
| Ratio of Water Demand/% | Fineness/% | Density/(g/cm3) | Compressive Strength Ratio/% | Specific Surface Area/(cm2/g) | Water Quantity/% | Packing Density/(g/cm3) | Normal Consistency/% |
|---|---|---|---|---|---|---|---|
| 94 | 10 | 2.1 | 78 | 3400 | 106 | 0.78 | 48 |
| Density/(g/cm3) | Specific Surface Area/(cm2/g) | Average Particle Size/μm | Bulk Density/(g/mL) |
|---|---|---|---|
| 1.6–1.7 | (20–28) × 10,000 | 0.1–0.3 | ≥0.67 |
| Species | Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| / | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | Na2O | SO3 | Loss on Ignition |
| P·O 42.5 cement | 61.536 | 15.404 | 4.430 | 0.724 | 4.906 | 0.043 | 2.755 | 2.243 |
| Silica fume | 0.568 | 97.35 | 0.337 | 0.414 | 0.003 | 0.101 | 0.192 | 2.810 |
| Fly ash | 1.5 | 58 | 30 | 2.8 | 4.3 | 3.2 | 0.8 | 3.310 |
| Species | Cement | Fly Ash | Silica Fume | Steel Fiber | Polycarboxylic Acid Series of Superplasticizer | Sea Sand | River Sand | Artificial Seawater | Fresh Water |
|---|---|---|---|---|---|---|---|---|---|
| SSUHPC | 0.6 | 0.25 | 0.15 | 0.19 | 0.03 | 1.4 | \ | 0.14 | \ |
| FRUHPC | 0.6 | 0.25 | 0.15 | 0.19 | 0.03 | \ | 1.4 | \ | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, T.; Shi, F.; Liu, X.; Zong, Y.; Hou, B.; Tian, H. Sulphate Corrosion Mechanism of Ultra-High-Performance Concrete (UHPC) Prepared with Seawater and Sea Sand. Polymers 2022, 14, 971. https://doi.org/10.3390/polym14050971
Sun X, Li T, Shi F, Liu X, Zong Y, Hou B, Tian H. Sulphate Corrosion Mechanism of Ultra-High-Performance Concrete (UHPC) Prepared with Seawater and Sea Sand. Polymers. 2022; 14(5):971. https://doi.org/10.3390/polym14050971
Chicago/Turabian StyleSun, Xin, Tianyu Li, Fangying Shi, Xiaoyan Liu, Yingxia Zong, Baorong Hou, and Huiwen Tian. 2022. "Sulphate Corrosion Mechanism of Ultra-High-Performance Concrete (UHPC) Prepared with Seawater and Sea Sand" Polymers 14, no. 5: 971. https://doi.org/10.3390/polym14050971