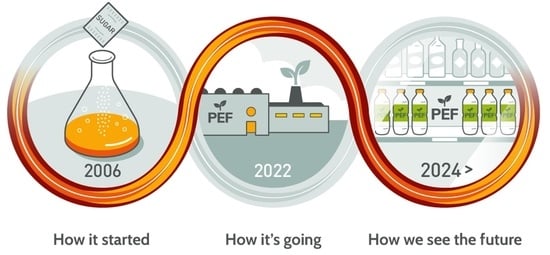
The Road to Bring FDCA and PEF to the Market
Abstract
:1. Avantium’s Goal: Bringing Disruptive Technologies to the Market
1.1. Avantium’s Coherent Portfolio of Technologies
1.2. Production of Demonstrators to Engage Partners at an Early Stage
1.3. Production at Pilot Plant Scale
1.4. The Importance of Patents and Patent Protection
1.5. Chemical Registration and Food Contact Approval
- Chemical registration in EU: Chemicals need to be registered according to the European Regulation often abbreviated by REACH (Registration, Evaluation, Authorization and Restriction of Chemicals). REACH requires the registration of the products produced and/or imported to the European Union including isolated intermediates when quantities are above 1 t per year, independently of the use. Depending on the tonnage band, different properties are evaluated and submitted to the European Chemical Agency (ECHA). Several toxicity studies need to be held, ensuring the safety of the production and the use of chemical substances. The requirements of the toxicity tests are very dependent on the tonnage band group that the company is producing and/or importing: (i) 1–10 t per year, (ii) 10–100 t per year, (iii) 100–1000 t per year, (iv) above 1000 t per year. The toxicity data obtained on each substance are shared within the group of producers/importers of that specific substance, under cost sharing principles. Exemptions of registration for research purposes are also possible via a PPORD (Product and Process Orientated Research and Development) application. When a company notifies ECHA with all the required information, the substance is then exempted from registration for the following 5 years, as long as the substance or article is only used for experimental trials and not used for commercial purposes, with no volume restriction.
- Polymer registration in EU: According to REACH polymers are exempted when the polymer producer has the registration or downstream use authorization for the monomers. This means that for the exemption of PEF, besides the registration of FDCA Avantium is also required to have a registration or a downstream user of MEG. Currently, the EU competent authorities are evaluating a regulation change to initiate the polymer registration processes and to define the group of polymers still exempted of registration when they are defined as polymer of low concern (PLC). Up to this day, REACH regulation has not been amended.
- Table 1(1): Base Polymers (Plastics);
- Table 1(2): Base Polymers (Coatings);
- Table 1(3): Minor Monomers;
- Table 2: Additives.
1.6. Strategic Routes for Monetising Breakthrough Technologies
- (i)
- Own and operate the technology;
- (ii)
- Applying the technology in partnerships or joint ventures;
- (iii)
- Licensing the technology to third parties;
- (iv)
- Or divesting the technology to third parties.
- (i)
- Greenfield, where the licensee starts constructing the FDCA plant from scratch; and
- (ii)
- Retrofitted purified terephthalic acid (PTA) plants where the existing PTA plant is being converted into an FDCA plant.
2. How the Technologies Developed over Time
2.1. Unlocking the Potential of a “Sleeping Giant”
- Step 1:
- Sugar dehydration. The catalytic dehydration (i.e., the removal of oxygen via water elimination) of plant-based sugars (high fructose syrup) in an alcohol, to make an alkoxymethyl furfural such as methoxymethyl furfural (MMF). Van Putten et al. has extensively reported about the chemistry involved in the conversion of carbohydrates into furanic compounds [3,33,34,35,36,37];
- Step 2:
- Oxidation the catalytic oxidation of an alkoxymethyl furfural (such as MMF) in acetic acid to make furan dicarboxylic acid (‘crude’ (c)FDCA). The similarities and differences of the conversions of para-xylene into terephthalic acid compared with the oxidation of RMF into FDCA have been extensively discussed by van der Waal et al. [38,39,40,41,42];
- Step 3:
- Step 4:
- Melt polymerization of FDCA and mono ethylene glycol (biobased MEG) to create the plant-based polymer, polyethylene furanoate (PEF) [5,22,48,49,50,51,52]. Typically, the melt polymerization is followed by a solid-state polymerization step to bring the polymer molecular weight to the desired values, depending on the target application(s) [53,54];
- Step 5:
- Step 6:

2.2. Sugar Dehydration into MMF/HMF and Oxidation into FDCA
2.3. Humins and Methyl Levulinate, Side-Products in the MMF Process
2.3.1. Humins
- (a)
- Optimizing the acid catalysed dehydration process targeting the minimization of humins production;
- (b)
- Adapting the acid catalysed dehydration process targeting the composition of the humins production. The use of an alcoholic solvent in the MMF process results in a highly viscous liquid humins (after solvent evaporation), instead of solid humins encountered in water-based dehydration systems;
- (c)
- As a base case, the conversion of humins into a heat and power source to satisfy a substantial part of the energy demand of the biorefinery is pursued;
- (d)
- A more favourable longer-term strategy is using the humins as a potential valuable, renewable feedstock for new biobased chemicals, biomaterials, and/or additives of interest.
2.3.2. Methyl Levulinate
2.4. Flywheel for Commercial Developments
2.5. Revised Scale-Up and Market Launch Strategy
3. How It Is Going: PEF Has the Potential to Revolutionize the Plastic Packaging Industry
3.1. The Need to Keep Fossil Resources in the Ground—And Only Use Carbon Sourced above the Ground
3.2. PEF Helps Tackle Climate Change and Addresses the Global Need to Reduce Plastic Waste
3.2.1. PEF in the Circular Economy
3.2.2. Re-Use
3.2.3. Mechanical Recycling: Closed Loop
3.2.4. Mechanical Recycling: Open Loop
3.2.5. Chemical Recycling
- The glycolysis route is the most implemented route for PET. In this process the addition of a glycol (typically MEG) and a transesterification catalyst at elevated temperatures converts PET into oligomers (also referred to as pre-polymers) such as bis-hydroxyethyl terephthalate (BHET). These pre-polymers can be fed in the melt polymerization process going back to PET (closed loop 3° recycle) but can also be applied as feedstock for other polymers like polyurethanes, thermoset resins, etc. (open loop 3° recycle). Little research has been published on the glycolysis of PEF, although Gabirondo et al. have demonstrated it is possible to apply a glycolysis process to PEF [113].
- The methanolysis route is considered to allow the best purification of the end products from contaminants. The addition of methanol leads to formation of dimethyl terephthalate (DMT) and MEG when starting with PET. Sipos et al. demonstrated that the methanolysis of PEF into dimethyl furanoate (DMF) and MEG can reach higher conversion rates than that of PET [40].
- In the hydrolysis route the polyester is broken down by reacting with water. Depending on the acidity this process can be sped up and carried out at milder conditions. The advantage of the hydrolysis route for PET is that it goes back to terephthalic acid (TA), with the downside that the purification of the TA is challenging. Sipos et al. have reported a yield >80% for a first acidic hydrolysis assessment on PEF, demonstrating that the principle can be applied to recover FDCA from PEF [61]. The alkaline hydrolysis of PET/PEF co-polyesters has been described by Vinnakota [114].
3.2.6. End-of-Life
3.3. Superior Functionality
| Property | PET (Amorphous) | PEF (Amorphous) | References |
|---|---|---|---|
| Molecule |  |  | |
| Density (amorphous) | 1.36 g/cm3 | 1.434 g/cm3 | [129,130,133,141] |
| Density (crystalline, calculated) | 1.455 g/cm3 | 1.565 g/cm3 | [129,130,142,143,144] |
| Melting temperature (Tm) | 250–270 °C | 210–230 °C | [56,109] |
| Glass transition temperature (Tg) | ~76 °C | ~88 °C | [145,146] |
| Crystallization time | 2–3 min | 20–30 min | [128,133,134,135,147] |
| E-modulus (ISO 527/1A, 1 mm/min) | 2.1–2.2 GPa | 3.6 GPa | [146] |
| Yield strength (ISO 527/1A, 10 mm/min) | 50–60 MPa | 90–100 MPa | [146] |
| O2 permeability * (@23 °C, 65% RH) | 2.5 cm3·mm/(m2∙24 h∙bar) | 0.23 cm3·mm/(m2∙24 h∙bar) | [146] |
| CO2 permeability * (@23 °C, 0% RH) | 23.6 cm3·mm/(m2∙24 h∙bar) | 1.6 cm3·mm/(m2∙24 h∙bar) | [146] |
| H2O permeability * (@38 °C, 90% RH) | 0.9 g∙mm/(m2∙24 h) | 0.36 g∙mm/(m2∙24 h) | [146] |
3.4. Sustainability
- The use of 100% renewable carbon in PEF instead of fossil carbon in PET for producing 250 mL bottles would result in significant reductions in greenhouse gas emissions (−33%) over the life cycle of the bottles.
- PEF bottles would also contribute to remarkably less finite resource consumption of fossil fuels (−45%) compared to that demanded by PET bottles.
- These impact potentials are two of the most relevant environmental impact categories in the current political agenda driving the transition from fossil to renewable carbon. This represents a significant benefit, because climate change and resource use were found to be the impact categories most heavily influencing the environmental impact of monolayer PEF bottles.
- Very significant is the lower pressure that the production of PEF bottles would put on abiotic resources (minerals and metals) in contrast to that caused during PET bottles production.
- The lower environmental footprint of the biobased alternative can be attributed, to a great extent, to the improved barrier and mechanical properties of PEF allowing for an overall 46% reduction in polymer usage in the manufacture of bottles. This is also combined with the biogenic nature of the emissions (from renewable carbon) that the biobased bottle would release upon incineration, which do not contribute additionally to climate change.
- The other evaluated impacts were found to be significantly less relevant and contribute to a minor extent to the total environmental impact of PEF bottles [149].
3.5. Disruptive Technologies need Trailblazers
- Have a good idea;
- Proof of Principle: Justify the idea by R&D in the lab;
- Conceptual Process Design (CPD) to assess the techno economics as well as to be able to perform an ex ante LCA assessment. CPD is also used to target the R&D on the aspects that have the largest impacts on costs as well as sustainability;
- Develop and execute IP strategy, assess Freedom to Operate the technology;
- Proof of Concept: run the process at pilot plant scale;
- The technology needs to be assessed for its ability to scale;
- Recyclability of the anticipated materials need to be proven;
- The right partners along the whole value-chain need to come on board, possible from step 3 onwards;
- For each application, pilot and pre-marketing studies need to be conducted at relevant scale thereby needing often ton(s) of material per pilot;
- Address all necessary regulatory aspects for building and operating a commercial plant as well as for the products made (a.o. REACH, Food Contact (EFSA) for Europe);
- Deliver the foundations for large-scale manufacturing, update techno-economic as well as LCA assessments;
- All while testing at every stage to ensure the appropriate safety and sustainability standards are met.
4. How Avantium Sees the Future: On the Edge of Commercialising PEF
- (a)
- to prove the process technology at scale, and
- (b)
- to demonstrate the commercial applications of FDCA and PEF.

5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Waal, J.C.; van Putten, R.-J.; Ras, E.-J.; Lok, M.; Gruter, G.-J.; Brasz, M.; de Jong, E. The High-Throughput research approach to Biorefineries—A powerful tool for studying the complexity of catalytic processes. Cellul. Chem. Technol. 2011, 45, 461–466. [Google Scholar]
- Gruter, G.J.; Sipos, L.; Dam, M.A. Accelerating research into bio-based FDCA-polyesters by using small scale parallel film reactors. Comb. Chem. High Throughput Screen 2012, 15, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.-J.; Soetedjo, J.N.M.; Pidko, E.A.; van der Waal, J.C.; Hensen, E.J.M.; de Jong, E.; Heeres, H.J. The dehydration of different ketoses and aldoses to 5-hydroxymethylfurfural. ChemSusChem 2013, 6, 1681–1687. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; Harmse, M.; van den Bovenkamp, H.H.; de Jong, E.; Heeres, H.J. A comparative study on the reactivity of various ketohexoses to furanics in methanol. ChemSusChem 2016, 9, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic acid (FDCA), a versatile building block for a very interesting class of polyesters. In Biobased Monomers, Polymers and Materials; Smith, P.B., Gross, R., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2012; Volume 1105, pp. 1–13. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Patel, M.K.; Faaij, A.P.C. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balances. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.; Gruter, G.-J.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5963–6098. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K.; de Jong, E.; de Sousa Dias, A.; van der Waal, J.C.; Grisel, R.J.H.; Huijgen, W.J.J. Fuels and plastics from lignocellulosic biomass via the furan pathway; A technical analysis. RSC Adv. 2014, 4, 3536–3549. [Google Scholar] [CrossRef]
- Grisel, R.J.H.; van der Waal, J.C.; de Jong, E.; Huijgen, W.J.J. Acid catalysed alcoholysis of wheat straw: Towards second generation furan-derivatives. Catal. Today 2014, 223, 3–10. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Patel, M.K.; Faaij, A.P.C. Fuels and plastics from lignocellulosic biomass via the furan pathway: An economic analysis. Biofuels Bioprod. Bioref. 2015, 9, 227–334. [Google Scholar] [CrossRef]
- Murcia Valderrama, M.A.; van Putten, R.-J.; Gruter, G.-J.M. PLGA barrier materials from CO2. The influence of lactide co-monomer on glycolic acid polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Murcia Valderrama, M.A.; van Putten, R.-J.; Davey, C.J.E.; Tietema, A.; Parsons, J.R.; Wang, B.; Gruter, G.-J.M. Biodegradation and Non-Enzymatic Hydrolysis of Poly(Lactic-co-Glycolic Acid) (PLGA12/88 and PLGA6/94). Polymers 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gruter, G.-J.M. Polyester Copolymer. PCT Patent Application WO2018211132A1, 22 November 2018. Available online: https://patentimages.storage.googleapis.com/ca/79/b9/ceec8cfb8d786c/WO2018211132A1.pdf (accessed on 7 January 2021).
- Wang, B.; Gruter, G.-J.M. Polyester Copolymer. PCT Patent Application WO2018211133A1, 22 November 2018. Available online: https://patentimages.storage.googleapis.com/31/75/c0/b414aa8400ddfe/WO2018211133A1.pdf (accessed on 7 January 2021).
- Kwon, Y.; de Jong, E.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic hydrogenation of 5-hydroxymethylfurfural in the absence and presence of glucose. ChemSusChem 2013, 6, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Schouten, K.-J.P.; van der Waal, J.C.; de Jong, E.; Koper, M.T.M. Perspective for electrocatalytic conversion of furanic compounds. ACS Catal. 2016, 6, 6704–6717. [Google Scholar] [CrossRef]
- Schouten, K.-J.; van der Waal, J.-C.; Varini, M.; Gruter, G.-J.M. Process for the Purification of a Carboxylic Acid-Containing Composition. PCT Patent Application WO2016186505A1, 24 November 2016. Available online: https://patentimages.storage.googleapis.com/44/7e/ce/864a56e2411d8e/WO2016186505A1.pdf (accessed on 30 December 2021).
- Schouten, K.-J.; Gruter, G.-J.M.; van der Waal, J.-C. Process for Treating a Furan-2,5-dicarboxylic Acid Composition. PCT Patent Application WO2018097725A1, 31 May 2018. Available online: https://patentimages.storage.googleapis.com/c5/43/e1/95734acebb9724/WO2018097725A1.pdf (accessed on 30 December 2021).
- Werpy, T.; Petersen, G.R. Top Value Added Chemicals from Biomass, Volume 1. Results of Screening for Potential Candidates from Sugars and Synthesis Gas. 2004. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 10 January 2022).
- De Jong, E.; Stichnothe, H.; Bell, G.; Jørgensen, H. Bio-Based Chemicals, a 2020 Update. IEA Bioenergy. ISBN 978-1-910154-69-4. 2020. Available online: http://task42.ieabioenergy.com/wp-content/uploads/2020/02/Bio-based-chemicals-a-2020-update-final-200213.pdf (accessed on 10 January 2022).
- Sipos, L.; Gruter, G.-J.M.; Kolstad, J.; Dam, M.A. Process for Preparing a Polymer Product Having a 2,5,-furandicarboxylic Moiety within the Polymer Backbone to Be Used in Bottle, Film or Fibre Applications. WO2013062408. 2 May 2013. Available online: https://patents.google.com/patent/WO2013062408A1/en?oq=WO2013062408 (accessed on 10 January 2022).
- Kolstad, J.; Gruter, G.-J.M. Process for the Preparation of a Fiber, a Fiber and a Yarn Made from Such a Fiber. WO2014204313. 24 December 2014. Available online: https://patents.google.com/patent/WO2014204313A1/en?oq=WO2014204313 (accessed on 10 January 2022).
- Van Berkel, J.G.; Kolstad, J.J. Process for Producing an Oriented Film Comprising Poly(ethylene-2,5-furandicarboxylate). WO2016032330. 3 March 2016. Available online: https://patents.google.com/patent/WO2016032330A1/en?oq=WO2016032330 (accessed on 10 January 2022).
- Inagaki, J.; Ito, K.; Shimizu, T.; Gyobu, S.; Morishige, C.; van Berkel, J.G. Polyester Film Containing Furandicarboxylic Acid. WO2017038092. 9 March 2017. Available online: https://patents.google.com/patent/WO2017038092A1/en?oq=WO2017038092 (accessed on 10 January 2022).
- Ikeda, Y.; Sugimoto, K.; Nakajima, H. Method for Producing PEF Yarn. WO2017043080. 16 March 2017. Available online: https://patentimages.storage.googleapis.com/20/1c/96/c186d7a9a22ab3/WO2017043080A1.pdf (accessed on 15 January 2022).
- Inagaki, J.; Numata, Y.; van Berkel, J.G. Laminated Polyester Film. WO2017115736. 6 July 2017. Available online: https://patents.google.com/patent/WO2017115736A1/en?oq=WO2017115736 (accessed on 5 January 2022).
- Inagaki, J.; Numata, Y.; van Berkel, J.G. Polyester Film. WO2017169553. 7 March 2017. Available online: https://patents.google.com/patent/JPWO2017169553A1/en?oq=WO2017169553 (accessed on 5 January 2022).
- Berny, B.J.; Acquasanta, F.; Flaciere, J.T.P.; van Berkel, J.G. Thermoformed Article of Poly(ethylene 2,5 furandicarboxylate) Polyester. WO2018097728. 31 May 2018. Available online: https://patents.google.com/patent/WO2018097728A1/en?oq=WO2018097728 (accessed on 4 January 2022).
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Product developments in the bio-based chemicals arena. Biofuels Bioprod. Bioref. 2012, 6, 606–624. [Google Scholar] [CrossRef]
- Deshan, A.D.K.; Atanda, L.; Moghaddam, L.; Rackemann, D.W.; Beltramini, J.; Doherty, W.O.S. Heterogeneous catalytic conversion of sugars into 2,5-furandicarboxylic acid. Front. Chem. 2020, 8, 659–682. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; de Sousa Dias, A.; de Jong, E. Furan based building blocks from carbohydrates. In Catalytic Process Development for Renewable Materials; Imhof, P., Van der Waal, J.C., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2013; pp. 81–117. ISBN 978-3-527-33169-7. [Google Scholar] [CrossRef]
- Van der Waal, J.C.; de Jong, E. Chemocatalytic processes for the production of bio-based chemicals from carbohydrates. In Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials; Luque, R., Balu, A.M., Eds.; CRC Press, Taylor & Francis: Baco Raton, FL, USA, 2013; pp. 223–266. ISBN 978-1-4665-5339-2. [Google Scholar] [CrossRef]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of 5-alkoxymethyl Furfural Ethers and Their Use. WO2007104514. 20 September 2007. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007104514 (accessed on 15 December 2021).
- De Sousa Dias, A.S.V.; Gruter, G.J.M.; van Putten, R.-J. Process for the Conversion of a Carbohydrate-Containing Feedstock. WO2012091570. 5 July 2012. Available online: https://patentimages.storage.googleapis.com/49/31/9e/711fbdfa18468a/WO2012091570A1.pdf (accessed on 28 December 2021).
- Van der Waal, J.C.; Mazoyer, E.; Baars, H.J.; Gruter, G.-J.M. From Terephthalic Acid to 2,5-Furandicarboxylic Acid: An Industrial Perspective. In Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives: Industrial Applications and Academic Perspectives; Shannon, S., Stahl, P., Alsters, L., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2016. [Google Scholar] [CrossRef]
- Muñoz de Diego, C.; Schammel, W.P.; Dam, M.A.; Gruter, G.-J.M. Method for the Preparation of 2,5-furandicarboxylic Acid and Esters Thereof. WO2011043660. 14 April 2011. Available online: https://patentimages.storage.googleapis.com/b4/0f/8e/105104a26e1184/WO2011043660A3.pdf (accessed on 22 December 2021).
- Muñoz de Diego, C.; Dam, M.A.; Gruter, G.-J.M. Methods for the Preparation of 2,5-furandicarboxylic Acid and for the Preparation of the Dialkylester of 2,5-furandicarboxylic Acid. WO2011043661. 14 April 2011. Available online: https://patentimages.storage.googleapis.com/d2/9a/b0/9fda67eb9f7860/WO2011043661A1.pdf (accessed on 5 January 2022).
- Mazoyer, E.; de Sousa Dias, A.S.V.; McKay, B.; Baars, R.; Vreeken, V.; Gruter, G.-J.M.; Sikkenga, D. Process for the Preparation of 2,5-furandicarboxylic Acid. WO2014163500. 9 October 2014. Available online: https://patents.google.com/patent/WO2014163500A1/en?oq=WO2014163500 (accessed on 5 January 2022).
- Baars, H.J.; Blank, J.H.; Kolstad, J.J.; de Sousa Dias, A.S.V. Process for Producing 2.5-furandicarboxylic Acid from Ethers of 5-hydroxymethylfufural. WO2021123189. 24 June 2021. Available online: https://patents.google.com/patent/WO2021123189A1/en?oq=WO2021123189 (accessed on 7 January 2022).
- Singh, J.; McKay, B.; de Sousa Dias, A.S.V.; Dam, M.A.; Gruter, G.-J.M. Process for Purifying an Acid Composition Comprising 2-formyl-furan-5-carboxylic Acid and 2,5-furandicarboxylic Acid. WO2015030590. 5 March 2015. Available online: https://patents.google.com/patent/WO2015030590A1/en?oq=WO2015030590 (accessed on 5 January 2022).
- Almeida, A.R.M.G.R.; Zieverink, M.M.P.; de Sousa Dias, A.S.V. Process for the Preparation of a Purified Acid Composition. WO2016195499. 8 December 2016. Available online: https://patents.google.com/patent/WO2016195499A1/en?oq=WO2016195499 (accessed on 19 December 2021).
- Almeida, A.R.M.G.R.; Zieverink, M.M.P.; de Sousa Dias, A.S.V.; Gonzalez, I. FDCA Purification by Hydrolysis and Hydrogenation. WO2016195500. 8 December 2016. Available online: https://patents.google.com/patent/WO2016195500A1/en?oq=WO2016195500 (accessed on 5 January 2022).
- Van Berkel, J.G.; Kolstad, J.J. Multilayer Container Comprising a Polyethylene Furanoate Layer. EP3705292. 9 September 2020. Available online: https://patentimages.storage.googleapis.com/c9/ab/05/aae6aad8ce0f6b/EP3705292A1.pdf (accessed on 22 December 2021).
- Kolstad, J.J.; de Sousa Dias, A.S.V.; Sijben, J.M.F.; Almeida, A.R.M.G.R.; Gonzalez Jimenez, I. Organic Acid and Thermal Treatment of Purified 2,5-Furandicarboxylic Acid. WO2021123203. 24 June 2021. Available online: https://patents.google.com/patent/WO2021123203A1/en?oq=+WO2021123203 (accessed on 10 January 2022).
- Kolstad, J.; Gruter, G.J.; Dam, R.; Wang, B.; van Berkel, J.; Pascke, E.; Schiavone, R.; Andrews, M. Polyester and Method for Preparing Such a Polyester. WO2015137807. 17 September 2015. Available online: https://patents.google.com/patent/WO2015137807A1/en?oq=WO2015137807 (accessed on 12 January 2022).
- Kolstad, J.J.; Wang, B.; Paschke, E.; Schiavone, R.; Andrews, M.; van Berkel, J.G. Polyester and Method for Preparing Such a Polyester. WO2015137805. 17 September 2015. Available online: https://patents.google.com/patent/WO2015137805A1/en?oq=WO2015137805 (accessed on 12 January 2022).
- Sipos, L. Process for the Preparation of a Polyester. WO2017048119. 23 March 2017. Available online: https://patents.google.com/patent/WO2017048119A1/en?oq=WO2017048119 (accessed on 22 December 2021).
- Acquasanta, F. Polyester Composition. WO2021089393. 14 May 2021. Available online: https://patents.google.com/patent/WO2021089393A1/en?oq=WO2021089393 (accessed on 5 January 2022).
- Nakatani, A.; Nakagawa, S.; Dam, M.A.; Kolstad, J.J. Method for Producing Polyester and Polyester Produced by Said Method. WO2021172215. 2 September 2021. Available online: https://patents.google.com/patent/WO2021172215A1/en?oq=WO2021172215 (accessed on 15 December 2021).
- Kolstad, J.; Gruter, G.J.; van Berkel, J.; Dam, R.; Schiavone, R.; Andrews, M. Process for Enhancing the Weight of a Polyester Composition. WO2015137806. 18 June 2015. Available online: https://patents.google.com/patent/WO2015137806A1/en (accessed on 15 December 2021).
- Van Berkel, J.G. Process for Enhancing the Molecular Weight of a Polyester by Solid State Polymerization. WO2017043974. 16 March 2017. Available online: https://patents.google.com/patent/WO2017043974A1/en?oq=WO2017043974 (accessed on 18 December 2021).
- A On-Stop Shop for Biodegradability Testing. 2016. Available online: https://www.ows.be/wp-content/uploads/2017/03/Article-Packaging-Europe-2016-1.pdf (accessed on 15 January 2022).
- Takarada, W.; Sugimoto, K.; Nakajima, H.; Visser, H.A.; Gruter, G.-J.M.; Kikutani, T. Melt-spun fibers from bio-based polyester–fiber structure development in high-speed melt spinning of poly(ethylene 2,5-furandicarboxylate) (PEF). Materials 2021, 14, 1172–1184. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sugimoto, K.; Dam, H. Fibre for Tire, Rubber/Fibre Complex, and Tire. WO2017043083. 16 March 2017. Available online: https://patents.google.com/patent/WO2017043083A1/en?oq=WO2017043083 (accessed on 10 January 2022).
- Ikeda, Y.; Sugimoto, K.; Nakajima, H. Fibre for Tire, Rubber/Fibre Complex, and Tire. WO2017043085. 16 March 2017. Available online: https://patents.google.com/patent/WO2017043085A1/en?oq=WO2017043085 (accessed on 10 January 2022).
- Ikeda, Y.; Sugimoto, K.; Nakajima, H.; van Berkel, J.G. Method for Producing PEF Yarn, PEF Yarn, and Tire. WO2017043082. 16 March 2017. Available online: https://patents.google.com/patent/WO2017043082A1/en?oq=WO2017043082 (accessed on 10 January 2022).
- Van Berkel, J.G.; Visser, H.A. Method for Fabricating a Container and the Container. WO2020013694. 16 January 2020. Available online: https://patents.google.com/patent/WO2020013694A1/en?oq=WO2020013694 (accessed on 18 December 2021).
- Sipos, L.; Olson, M. Process for the Depolymerization of a Furan Dicarboxylate Containing Polyester. WO2012091573. 27 June 2013. Available online: https://patents.google.com/patent/WO2012091573A9/en?oq=WO2012091573 (accessed on 15 January 2022).
- Sipos, L.; Gruter, G.-J.M.; Kolstad, J.J.; Degand, G.; Jacquel, N.; Saint-Loup, R.; Dam, M.A.; Schroeder, J.D. Polyesters Comprising 2,5-furandicarboxylic Acids and Saturated Diol Units Having a High Glass Transition Temperature. WO2015142181. 24 September 2015. Available online: https://patents.google.com/patent/WO2015142181A1/en?oq=WO2015142181 (accessed on 12 December 2021).
- Gandini, A. Furans as offsprings of sugars and polysaccharides and progenitors of an emblematic family of polymer siblings. In Green Polymeris ation Methods: Renewable Starting Materials, Catalysis and Waste Reduction; Wiley-VCH Weinheim, Wiley Online Library: Weinheim, Germany, 2011; Volume 29. [Google Scholar] [CrossRef]
- Tsanaktsis, V.; Vouvoudi, E.; Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully biobased superpolymers of 2,5-furandicarboxylic acid with different functional properties: From rigid to flexible, high performant packaging materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Kamel, C. High-Performance Polymeric Applications for FDCA beyond PEF. Master’s Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2020. Available online: https://www.google.nl/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjqmtbYmvL0AhXL_qQKHQt0Au0QFnoECAQQAQ&url=https%3A%2F%2Fscripties.uba.uva.nl%2Fdownload%3Ffid%3D678606&usg=AOvVaw1lgRo7wqDUuriqjfXjiS0R (accessed on 12 January 2022).
- Kainulainen, T.P.; Erkkilä, P.; Terttu, I.; Hukka, T.I.; Sirviö, J.A.; Heiskanen, J.P. Application of furan-based dicarboxylic acids in bio-derived dimethacrylate resins. ACS Appl. Polym. Mater. 2020, 2, 3215–3225. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the properties of furandicarboxylic acid-based polyesters with copolymerization: A review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Qi, Z.; He, L.; Peng, L. Progress in the synthesis and properties of 2,5-furan dicarboxylate based polyesters. BioResources 2020, 15, 4502–4527. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Schneider, D.F.; Schreuder, I. Synthesis and evaluation of dialkyl furan-2,5-dicarboxylates as plasticizers for PVC. J. Appl. Polym. Sci. 1994, 53, 1785–1793. [Google Scholar] [CrossRef]
- Grass, M.; Becker, H.G. 2,5-Furan Dicarboxylate Derivatives, and Use Thereof as Plasticizers. U.S. Patent Application US20120220507, 3 August 2012. Available online: https://patentimages.storage.googleapis.com/68/d9/0d/e7c2900568cf67/US20120220507A1.pdf (accessed on 15 January 2022).
- Van Vugt-Lussenburg, B.M.A.; van Es, D.S.; Naderman, M.; le Notre, J.; van der Klis, F.; Brouwer, A.; van der Burg, B. Endocrine activities of phthalate alternatives; assessing the safety profile of furan dicarboxylic acid esters using a panel of human cell based reporter gene assays. Green Chem. 2020, 22, 1873–1883. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; Winkelman, J.G.M.; Keihan, F.; van der Waal, J.C.; de Jong, E.; Heeres, H.J. Experimental and modeling studies on the solubility of d-Arabinose, d-Fructose, d-Glucose, d-Mannose, Sucrose and d-Xylose in methanol and methanol–water mixtures. Ind. Eng. Chem. Res. 2014, 53, 8285–8290. [Google Scholar] [CrossRef]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Van Zandvoort, I.; Koers, E.J.; Weingarth, M.; Bruijnincx, P.C.A.; Baldus, M.; Weckhuysen, B.M. Structural characterization of 13 C-enriched humins and alkali-treated 13 C humins by 2D solid-State NMR. Green Chem. 2015, 17, 4383–4392. [Google Scholar] [CrossRef] [Green Version]
- Sangregorio, A.; Guigo, N.; Van Der Waal, J.C.; Sbirrazzuoli, N. All ‘green’ composites comprising flax fibres and humins’ resins. Compos. Sci. Technol. 2019, 171, 70–77. [Google Scholar] [CrossRef]
- Sangregorio, A.; Muralidhara, A.; Marlair, G.; Angelici, C.; de Jong, E.; Sbirrazzuoli, N.; Guigo, N. Natural fibre composites with furanic resins. Comparison between polyfurfuryl alcohol and humins from sugar conversion. Compos. Part C Open Access 2021, 4, 100109. [Google Scholar] [CrossRef]
- Mija, A.; Pin, J.M.; van der Waal, J.C.; Guigo, N.; de Jong, E. Humins as promising material for producing sustainable carbohydrate-derived building materials. Constr. Build. Mater. 2017, 139, 594–601. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Rodriguez-Castellon, E.; Van Der Waal, J.C.; Luque, R. Benign-by-design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef] [Green Version]
- Pin, J.-M.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; van der Waal, J.C.; de Jong, E. Valorization of bio-refinery side-stream products: From humins to biobased composites. ACS Sustain. Chem. Eng. 2014, 2, 2182–2190. [Google Scholar] [CrossRef]
- De Jong, E.; van der Waal, J.C.; Guigo, N.; Pin, J.M. Composition Comprising Furfuryl Alcohol. WO2015088341. 18 June 2015. Available online: https://patents.google.com/patent/WO2015088341A1/en?oq=WO2015088341 (accessed on 15 December 2021).
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Marlair, G.; Angelici, C.; de Jong, E.; Sbirrazzuoli, N. Humins-based resin for wood modification and properties improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef] [Green Version]
- Feijen, J.; van Klink, G.P.M.; de Jong, E.; Schmid, A.; Deen, N.; Boot, M.D. Spray combustion analysis of humins. SAE Tech. Pap. 2017, 24–0119. [Google Scholar] [CrossRef] [Green Version]
- Muralidhara, A.; Tosi, P.; Mija, A.; Sbirrazzuoli, N.; Len, C.; Engelen, V.; de Jong, E.; Marlair, G. Insights on thermal hazards of humins in support of their sustainable use in advanced biorefineries. ACS Sustain. Chem. Eng. 2018, 6, 16692–16701. [Google Scholar] [CrossRef]
- Muralidhara, A.; Bado-Nilles, A.; Marlair, G.; Engelen, V.; Len, C.; Pandard, P. Humins in the environment: Early stage insights on ecotoxicological aspects. Biofuels Bioprod. Bioref. 2019, 13, 464–470. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; Van Der Waal, J.C.; Sbirrazzuoli, N. Humins from biorefineries as thermoreactive macromolecular systems. ChemSusChem 2018, 11, 4246–4255. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; de Jong, E.; Sbirrazzuoli, N. Kinetics and chemorheological analysis of cross-linking reactions in humins. Polymers 2019, 11, 1804. [Google Scholar] [CrossRef] [Green Version]
- Mija, A.C.; de Jong, E.; van der Waal, J.C.; van Klink, G.P.M. Humins-Containing Foam. WO2017074183. 22 June 2017. Available online: https://patents.google.com/patent/WO2017074183A8/en?oq=WO2017074183.AKC037 (accessed on 15 December 2021).
- Tosi, P.; van Klink, G.P.M.; Celzard, A.; Fierro, V.; Vincent, L.; de Jong, E.; Mija, A. Auto-crosslinked rigid foams derived from biorefinery by-products. ChemSusChem 2018, 11, 2797–2809. [Google Scholar] [CrossRef] [PubMed]
- Tosi, P.; van Klink, G.P.M.; Hurel, C.; Lomenech, A.; Celzard, A.; Fierro, V.; Delgado-Sanchez, C.; Mija, A. Investigating the properties of humins foams, the porous carbonaceous materials derived from biorefinery by-products. Appl. Mater. Today 2020, 20, 100622. [Google Scholar] [CrossRef]
- Dinu, R.; Mija, A. Cross-linked polyfuran networks with elastomeric behaviour based on humins biorefinery by-products. Green Chem. 2019, 21, 6277–6289. [Google Scholar] [CrossRef] [Green Version]
- Montané, X.; Dinu, R.; Mija, A. Synthesis of resins using epoxies and humins as building blocks: A mechanistic study based on in-situ FT-IR and NMR spectroscopies. Molecules 2019, 24, 4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarutti, C.; Dinu, R.; Mija, A. Biorefinery byproducts and epoxy biorenewable monomers: A structural elucidation of humins and triglycidyl ether of phloroglucinol cross-linking. Biomacromolecules 2019, 21, 517–533. [Google Scholar] [CrossRef] [Green Version]
- Sangregorio, A.; Guigo, N.; Vincent, L.; de Jong, E.; Sbirrazzuoli, N. Furanic humins from biorefinery as biobased binder for bitumen. Polymers 2022. submitted. [Google Scholar]
- Van Klink, G.P.M.; de Jong, E. Asphalt Composition Comprising Humins Obtained from Dehydration of Carbohydrates. PCT Patent Application WO2018135941Al, 26 July 2018. Available online: https://patents.google.com/patent/WO2018135941A1/en?oq=WO2018135941Al (accessed on 10 January 2022).
- Van der Waal, J.C.; de Jong, E. Avantium chemicals: The high potential for the levulinic product tree. In Industrial Biorenewables, a Practical Viewpoint; Domínguez de María, P., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 97–120. [Google Scholar] [CrossRef]
- Filiciotto, L.; Balu, A.M.; Van Der Waal, J.C.; Luque, R. Catalytic insights into the production of biomass-derived side products methyl levulinate, furfural and humins. Catal. Today 2018, 302, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; McCormick, R.L.; Luecke, J.; de Jong, E.; van der Waal, J.C.; van Klink, G.P.M.; Boot, M.D. Anti-knock quality of sugar derived levulinic esters and cyclic ethers. Fuel 2017, 202, 414–425. [Google Scholar] [CrossRef]
- Byrne, F.P.; Clark, J.H.; Angelici, C.; de Jong, E.; Farmer, T.J. Greenness assessment and synthesis for the bio-based production of the solvent 2,2,5,5-tetramethyloxolane (TMO). Sustain. Chem. 2021, 2, 392–406. [Google Scholar] [CrossRef]
- El Ouahabi, F.; Polyakov, M.; van Klink, G.P.M.; Wohlrab, S.; Tin, S.; de Vries, J.G. Highly efficient and atom economic route for the production of methyl acrylate and acetic acid from a biorefinery side stream. ACS Sustain. Chem. Eng. 2020, 8, 1705–1708. [Google Scholar] [CrossRef]
- El Ouahabi, F.; Smit, W.; Angelici, C.; Polyakov, M.; Rodemerck, U.; Fischer, C.; Kalevaru, V.N.; Wohlrab, S.; Tin, S.; van Klink, G.P.M.; et al. Conversion of biomass-derived Methyl Levulinate to Methyl Vinyl Ketone. ACS Sustain. Chem. Eng. 2022, 10(2), 766–775. [Google Scholar] [CrossRef]
- European PET Bottle Platform Technical Opinion. Synvina = Poly(ethylene 2,5-furandicarboxylate) Resin. 2017. Available online: https://www.epbp.org/download/319/interim-approval-synvinas-polyethylene-25-furandicarboxylate-or-pef (accessed on 15 December 2021).
- Avantium Takes a Positive Final Investment Decision on the construction of Its FDCA Flagship Plant. 9 December 2021. Available online: https://www.avantium.com/wp-content/uploads/2021/12/20211209-Press-Release-Avantium-takes-a-positive-Final-Investment-Decision-on-the-Construction-of-its-FDCA-Flagship-Plant.pdf (accessed on 18 December 2021).
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_Full_Report.pdf (accessed on 18 December 2021).
- Hamilton, L.A.; Feit, S.; Kelso, M.; Muffett, C.; Rubright, S.M.; Bernhardt, C.; Labbé-Bellas, R. Plastic & Climate. The Hidden Costs of a Plastic Planet. 2019, pp. 1–108. Available online: https://www.ciel.org/wp-content/uploads/2019/05/Plastic-and-Climate-FINAL-2019.pdf. (accessed on 18 December 2021).
- Carus, M.; Dammer, L.; Raschka, A.; Skoczinski, P.; vom Berg, C. Renewable Carbon—Key to a Sustainable and Future-Oriented Chemical and Plastic Industry. 2020. Available online: https://renewable-carbon-initiative.com/wp-content/uploads/2020/09/20-09-21_Paper_12-on-Renewable-Carbon.pdf. (accessed on 18 December 2021).
- Papageorgiou, D.G.; Tsetsou, I.; Ioannidis, R.O.; Nikolaidis, G.N.; Exarhopoulos, S.; Kasmi, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. A new era in engineering plastics: Compatibility and perspectives of sustainable alipharomatic poly(ethylene terephthalate)/poly(ethylene 2,5-furandicarboxylate) blends. Polymers 2021, 13, 1070. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.A. PET and PEF. A Combination Fit for Future Sustainable Barrier Packaging Solutions. ComPETence Magazine. One:20. 2020. Available online: https://www.petnology.com/competence-magazine/news-details/pet-and-pef.html (accessed on 12 December 2021).
- Acquasanta, F.; Visser, H.A.; Langius, B. PEF as a Multilayer Barrier Technology: A Sustainable Way to Enable Long Shelf Life in PET Bottles. ComPETence Magazine. Two:20. 2020. Available online: https://www.petnology.com/competence-magazine/news-details/pef-as-a-multilayer-barrier-technology-a-sustainable-way-to-enable-long-shelf-life-in-pet-bottles.html (accessed on 12 December 2021).
- Eaves, E.; Acquasanta, F. Improving the Sustainability and Performance of Multilayer Barrier Containers with PEF. ComPETence Magazine. 2021. One:21. Available online: https://www.petnology.com/competence-magazine/news-details/improving-the-sustainability-and-performance-of-multilayer-barrier-containers-with-pef.html (accessed on 12 December 2021).
- Siddiqui, M.N.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Chemical recycling of pet in the presence of the bio-based polymers, PLA, PHB and PEF: A review. Sustainability 2021, 13, 10528. [Google Scholar] [CrossRef]
- Gabirondo, E.; Melendez-Rodriguez, B.; Arnal, C.; Lagaron, J.M.; Martínez de Ilarduya, A.; Sardon, H.; Torres-Giner, S. Organocatalyzed closed-loop chemical recycling of thermo-compressed food packaging films of Poly(ethylene furanoate). ChemRxiv 2020, 15. [Google Scholar] [CrossRef]
- Vinnakota, K. Chemical Recycling of Poly(Ethylene Terephthalate) and Its Co-Polyesters with 2, 5-furandicarboxylic Acid Using Alkaline Hydrolysis. Master’s Thesis, University of Toledo, Toledo, OH, USA, 2018. Available online: https://etd.ohiolink.edu/apexprod/rws_olink/r/1501/10?clear=10&p10_accession_num=toledo1535106967389994 (accessed on 15 January 2022).
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K.; et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef] [Green Version]
- Hiraga, K.; Taniguchi, I.I.; Yoshida, S.; Kimura, Y.; Oda, K. Biodegradation of waste PET: A sustainable solution for dealing with plastic pollution. EMBO Rep. 2019, 20, e49365. [Google Scholar] [CrossRef]
- Pellis, A.; Haernvall, K.; Pichler, C.M.; Ghazaryan, G.; Breinbauer, R.; Guebitz, G.M. Enzymatic hydrolysis of poly(ethylene furanoate). J. Biotechnol. 2016, 235, 47–53. [Google Scholar] [CrossRef]
- Aspalter, K. How Packaging Contributes to Food Waste Prevention. How Packaging Contributes to Food Waste Prevention. Denkstatt Sustainable Thinking. 2017, p. 42. Available online: https://denkstatt.eu/publications/ (accessed on 10 January 2022).
- Hoy, P. Reducing Food Waste by Extending Product Life. WRAP Final Report. 2015. Available online: https://refreshcoe.org/wp-content/uploads/2017/06/Product-Life-Report_Mar2015.pdf (accessed on 10 January 2022).
- Gooch, M.; Bucknell, D.; LaPlain, D.; Whitehead, P.; Marenick, N. Less Food Loss and Waste, Less Packaging Waste. National Zero Waste Council. 2020. Available online: http://www.nzwc.ca/Documents/FLWpackagingReport.pdf (accessed on 10 January 2022).
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Muburak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal and mechanical properties of Poly(ethylene furanoate) compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Karvan, O.; Johnson, J.R.; Kriegel, R.M.; Koros, W.J. Oxygen sorption and transport in amorphous poly(ethylene furanoate). Polymer 2014, 55, 4748–4756. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Muburak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 1: Equilibrium sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Muburak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly (ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Burgess, S.K.; Wenz, G.B.; Kriegel, R.M.; Koros, W.J. Penetrant transport in semicrystalline poly(ethylene furanoate). Polymer 2016, 98, 305–310. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro-Claro, P.J.A.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Sousa, A.F. Inside PEF: Chain conformation and dynamics in crystalline and amorphous domains. Macromolecules 2018, 51, 3515–3526. [Google Scholar] [CrossRef]
- Van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sbirrazzuoli, N. Biaxial orientation of poly(ethylene 2,5-furandicarboxylate): An explorative study. Macromol. Mater. Eng. 2018, 303, 1700507. [Google Scholar] [CrossRef]
- Mao, Y.; Bucknall, D.G.; Kriegel, R.M. Synchrotron X-ray scattering study on amorphous poly(ethylene furanoate) under uniaxial deformation. Polymer 2018, 139, 60–67. [Google Scholar] [CrossRef]
- Mao, Y.; Bucknall, D.G.; Kriegel, R.M. Simultaneous WAXS/SAXS study on semi-crystalline Poly(ethylene furanoate) under uniaxial stretching. Polymer 2018, 143, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Forestier, E.; Combeaud, C.; Guigo, N.; Monge, G.; Haudin, J.-M.; Sbirrazzuoli, N.; Billon, N. Strain-induced crystallization of poly(ethylene 2,5-furandicarboxylate). Mechanical and crystallographic analysis. Polymer 2020, 187, 122126. [Google Scholar] [CrossRef] [Green Version]
- Forestier, E.; Combeaud, C.; Guigo, N.; Sbirrazzuoli, N.; Billon, N. Understanding of strain-induced crystallization developments scenarios for polyesters: Comparison of poly(ethylene furanoate), PEF, and poly(ethylene terephthalate), PET. Polymer 2020, 203, 122755. [Google Scholar] [CrossRef]
- Van Berkel, J.G.; Guigo, N.; Kolstad, J.J.; Sipos, L.; Wang, B.; Dam, M.A.; Sbirrazzuoli, N. Isothermal crystallization kinetics of Poly(Ethylene 2,5-Furandicarboxylate). Macromol. Mater. Eng. 2015, 300, 466–474. [Google Scholar] [CrossRef]
- Codou, A.; Guigo, N.; van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Non-isothermal crystallization kinetics of biobased poly(ethylene 2,5-furandicarboxylate) synthesized via direct esterification process. Macromol. Chem. Phys. 2014, 215, 2065–2074. [Google Scholar] [CrossRef]
- Guigo, N.; Van Berkel, J.; de Jong, E.; Sbirrazzuoli, N. Modelling the non-isothermal crystallization of polymers: Application to poly(ethylene 2,5-furandicarboxylate). Thermochim. Acta 2017, 650, 66–75. [Google Scholar] [CrossRef]
- Martino, L.; Guigo, N.; van Berkel, J.B.; Kollstad, J.J.; Sbirrazzuoli, N. Nucleation and self-nucleation of bio-based poly(ethylene 2,5-furandicarboxylate) probed by fast scanning calorimetry. Macromol. Chem. Eng. 2016, 301, 586–596. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J.M. Isothermal crystallization and structural characterization of PEF. Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Menager, C.; Guigo, N.; Martino, L.; Sbirrazzuoli, N.; Visser, H.A.; Boyer, S.A.E.; Billon, N.; Monge, G.; Combeaud, C. Strain induced crystallization in biobased Poly(ethylene 2,5-furandicarboxylate) (PEF); conditions for appearance and microstructure analysis. Polymer 2018, 158, 364–371. [Google Scholar] [CrossRef]
- Forestier, E.; Combeaud, C.; Guigo, N.; Corvec, G.; Pradille, C.; Sbirrazzuoli, N.; Billon, N. Comparative analysis of the mechanical behaviour of PEF and PET uniaxial stretching based on the time/temperature superposition principle. Polymers 2021, 13, 3295. [Google Scholar] [CrossRef]
- Stoclet, G.; Lefebvre, J.M.; Yeniad, B.; Gobius du Sart, G.; de Vos, S. On the strain-induced structural evolution of Poly(ethylene-2,5-furanoate) upon uniaxial stretching: An in-situ SAXS-WAXS study. Polymer 2018, 134, 227–241. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Physical aging in amorphous poly (ethylene furanoate): Enthalpic recovery, density, and oxygen transport considerations. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 389–399. [Google Scholar] [CrossRef]
- Thompson, A.; Woods, D. Density of amorphous PolyEthylene Terephthalate. Nature 1955, 176, 78–79. [Google Scholar] [CrossRef]
- Daubeny, R.D.; Bunn, C.W. The Crystal Structure of Polyethylene Terephthalate. Proc. R. Soc. Lond. Ser. A 1954, 226, 531–542. [Google Scholar] [CrossRef]
- Kazaryan, L.G.; Medvedeva, F.M. X-ray study of poly (ethylene furan-2, 5-dicarboxylate) structure. Vysokomol. Soedin. Ser. B 1968, 10, 305. [Google Scholar]
- Codou, A.; Moncel, M.; van Berkel, J.B.; Guigo, N.; Sbirrazzuoli, N. Glass transition dynamics and cooperativity length of poly(ethylene 2,5-furandicarboxylate) compared to poly(ethylene terephthalate). Phys. Chem. Chem. Phys. 2016, 18, 16647–16658. [Google Scholar] [CrossRef] [PubMed]
- Avantium Technical Datasheet G90. Internal Avantium Document Which Can Be Made Available upon Reasonable Request. 2020. Available online: https://www.avantium.com/ (accessed on 15 January 2022).
- Van Berkel, J.G.; Guigo, N.; Visser, H.A.; Sbirrazzuoli, N. Chain structure and molecular weight dependent mechanics of PEF compared to PET. Macromolecules 2018, 51, 8539–8549. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- PEF Bottles—A Sustainable Packaging Material. ISO Certified LCA of Avantium’s PEF Products. LCA Brochure Available on the Avantium Site. Available online: https://www.avantium.com (accessed on 1 January 2022).
- Muralidhara, A.; de Jong, E.; Visser, H.A.; Gruter, G.-J.M.; Len, C.; Bertrand, J.-P.; Marlair, G. The fire propagation behaviour of some biobased furanic compounds with a focus on the polymer PEF. ACS Omega 2022, in press. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. https://doi.org/10.3390/polym14050943
de Jong E, Visser HA, Dias AS, Harvey C, Gruter G-JM. The Road to Bring FDCA and PEF to the Market. Polymers. 2022; 14(5):943. https://doi.org/10.3390/polym14050943
Chicago/Turabian Stylede Jong, Ed, Hendrikus (Roy) A. Visser, Ana Sousa Dias, Clare Harvey, and Gert-Jan M. Gruter. 2022. "The Road to Bring FDCA and PEF to the Market" Polymers 14, no. 5: 943. https://doi.org/10.3390/polym14050943