Fabrication of Highly Photostable Polystyrene Films Embedded with Organometallic Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Synthesis of Metal Complexes
2.3. Films Preparation and Irradiation
3. Results and Discussion
3.1. Synthesis of Ibuprofen–Metal Complexes
3.2. Investigation of Photostability of PS Using FTIR Spectrometry
3.3. Investigation of Photostability of PS Using Weight Loss Analysis
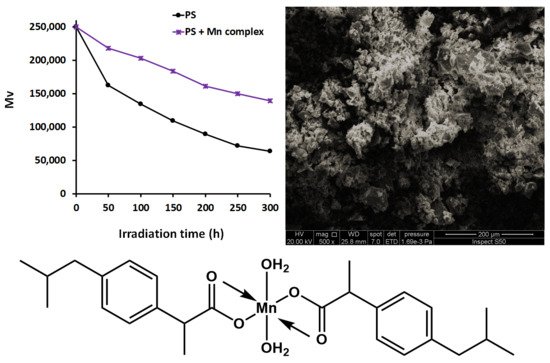
3.4. Investigation of Photostability of PS Using Molecular Weight
3.5. Investigation of Photostability of PS Using Surface Morphology
3.6. Proposed Mechanisms for PS Photostability Using Ibuprofen–Metal Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Marfa, I.M. Pyrolysis of polystyrene waste: A review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Maul, J.; Frushour, B.G.; Kontoff, J.R.; Eichenauer, H.; Ott, K.-H.; Schade, C. Polystyrene and Styrene Copolymers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007; pp. 477–484. [Google Scholar]
- Gary, J.E. Polystyrene: Properties, Performance and Applications; Nova Science: New York, NY, USA, 2011; pp. 1–198. [Google Scholar]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym. Degrad. Stab. 2006, 91, 2163–2170. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Structure and physical properties of syndiotactic polypropylene: A highly crystalline thermoplastic elastomer. Prog. Polym. Sci. 2006, 31, 145–237. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.R.; Turnbull, A. Weathering of polymers: Mechanisms of degradation and stabilization: Testing strategies and modelling. J. Mater. Sci. 1994, 29, 584–613. [Google Scholar] [CrossRef]
- Rabek, J.; Ranby, B. Photodegradation, Photooxidation and Photostabilization of Polymer; John Wiley: New York, NY, USA, 1975; pp. 501–554. [Google Scholar]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Mailhot, B.; Gardette, J.L. Polystyrene photooxidation. 1. Identification of the IR-absorbing photoproducts formed at short and long wavelengths. Macromolecules 1992, 25, 4119–4126. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 599–603. [Google Scholar]
- Pinto, L.F.A.; Goi, B.E.; Schmitt, C.C.; Neumann, M.G. Photodegradation of polystyrene films containing UV-visible sensitizers. J. Res. Updates Polym. Sci. 2013, 2, 39–47. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Torikai, A.; Kobatake, T.; Okisaki, F.; Shuyama, H. Photodegradation of polystyrene containing flame-retardants: Wavelength sensitivity and efficiency of degradation. Polym. Degrad. Stab. 1995, 50, 261–267. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Hameed, A.S.; Ahmed, A. Evaluation of the use of polyphosphates as photostabilizers and in the formation of ball-like polystyrene materials. J. Polym. Res. 2019, 26, 161. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R.; El-Hiti, G.A.; Yusop, R.M. Spectroscopic and photochemical stability of polystyrene films in the presence of metal complexes. J. Taibah Univ. Sci. 2017, 11, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff bases microspheres as photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, A.A.; Al-Tikrity, E.T.B.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Effect of ultraviolet irradiation on polystyrene containing cephalexin Schiff bases. Polymers 2021, 13, 2982. [Google Scholar] [CrossRef]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Yousif, E. SEM morphological analysis of irradiated polystyrene film doped by a Schiff base containing a 1,2,4-triazole ring system. Appl. Petrochem. Res. 2019, 9, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Goldshtein, J.; Margel, S. Synthesis and characterization of polystyrene/2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethylethyl)-4-methylphenol composite microspheres of narrow size distribution for UV irradiation protection. Colloid Polym. Sci. 2011, 289, 1863–1874. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N. New stabilizer for polystyrene based on 2-N-salicylidene-5-(substituted)-1,3,4-thiadiazole compounds. J. Saudi Chem. Soc. 2011, 16, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Rabie, S.T.; Ahmed, A.E.; Sabaa, M.W.; Abd El-Ghaffar, M.A. Maleic diamides as photostabilizers for polystyrene. J. Ind. Eng. Chem. 2013, 19, 1869–1878. [Google Scholar] [CrossRef]
- Torikai, A.; Takeuchi, T.; Fueki, K. Photodegradation of polystyrene and polystyrene containing benzophenone. Polym. Photochem. 1983, 3, 307–320. [Google Scholar] [CrossRef]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than 2 years: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef]
- Pierce, C.A.; Voss, B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: A meta-analysis and qualitative review. Ann. Pharmacother. 2010, 44, 489–506. [Google Scholar] [CrossRef]
- Alcock, N.W.; Culver, J.; Roe, S.M. Secondary bonding. Part 15. Influence of lone pairs on coordination: Comparison of diphenyl-tin (IV) and –tellurium (IV) carboxylates and dithiocarbamates. J. Chem. Soc. Dalton Trans. 1992, 9, 1477–1484. [Google Scholar] [CrossRef]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refat, M.S.; El-Metwaly, N.M. Spectroscopic and fluorescence studies on Mn(II), Co(II), Ni(II) and Cu(II) complexes with NO donor fluorescence dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 81, 215–227. [Google Scholar] [CrossRef]
- Rasyda, Y.A.; Rahardjo, S.B.; Nurdiyah, F. Synthesis and characterization complex nickel(II) with diphenylamine. IOP Conf. Ser. Mater. Sci. Eng. 2019, 578, 12008. [Google Scholar] [CrossRef]
- Mohammed, A.; Yousif, E.; El-Hiti, G.A. Synthesis and use of valsartan metal complexes as media for carbon dioxide storage. Materials 2020, 13, 1183. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization; Sharma, S., Ed.; Springer: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Biazar, E.; Zeinali, R.; Montazeri, N.; Pourshamsian, K.; Behrouz, M.; Asefnejad, A.; Khoshzaban, A.; Shahhosseini, G.; Najafabadi, M.S.; Abyani, R.; et al. Cell engineering: Nanometric grafting of poly-N-isopropylacrylamide onto polystyrene film by different doses of gamma radiation. Int. J. Nanomed. 2010, 5, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Sharma, T.; Aggarwal, S.; Kumar, S.; Mittal, V.K.; Kalsi, P.C.; Manchanda, V.K. Effect of gamma irradiation on the optical properties of CR-39 polymer. J. Mater. Sci. 2007, 42, 1127–1130. [Google Scholar] [CrossRef]
- Lucki, J.; Rånby, B. Photo-oxidation of polystyrene–Part 2: Formation of carbonyl groups in photo-oxidised polystyrene. Polym. Degrad. Stab. 1979, 1, 165–179. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Sonsuk, M.; Wittayapichet, S.; Prasassarakich, P.; Vejjanukroh, P.-C. Degradation of styrene-g-cassava starch filled polystyrene plastics. Polym. Degrad. Stab. 1999, 66, 323–334. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation: Mechanisms and Experimental Methods; Springer Science & Business Media: Berlin, Germany, 1994; pp. 1–664. [Google Scholar]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Allcock, H.R.; Lampe, F.W.; Mark, J.E. Contemporary Polymer Chemistry, 3rd ed.; Pearson Prentice-Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Erlandsson, B.; Albertsson, A.-C.; Karlsson, S. Molecular weight determination in degraded oxidizable and hydrolyzable polymers giving deviation from accurate using calibration and the Mark-Houwink-Sakaruda (MHS) equation. Polym. Degrad. Stab. 1997, 57, 15–23. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R.; Noaman, R. Photostabilization of Polystyrene Films: Photostabilization Activity of Polystyrene; Lambert Academic Publishing: Beau Bassin, Mauritius, 2014; p. 56. [Google Scholar]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, T.; Guttmann, P.; Schmidt, O.; Müller-Buschbaum, P.; Stamm, M.; Schönhense, G.; Schmahl, G. Microscopy of thin polymer blend films of polystyrene and poly-n-butyl-methacrylate. AIP Conf. Proc. 2000, 507, 245. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates in the photo-oxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Shinato, K.W.; Huang, F.; Jin, Y. Principle and application of atomic force microscopy (AFM) for nanoscale investigation of metal corrosion. Corros. Rev. 2020, 38, 423–432. [Google Scholar] [CrossRef]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. J. Appl. Polym. Sci. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Reginald, R.J.; Carson Meredith, J.C. Measurement of polyamide and polystyrene adhesion with coated-tip atomic force microscopy. J. Colloid Interface Sci. 2007, 314, 52–62. [Google Scholar] [CrossRef]
- Goudy, A.; Gee, M.L.; Biggs, S.; Underwood, S. Atomic force microscopy study of polystyrene latex film morphology: Effects of aging and annealing. Langmuir 1995, 11, 4454–4459. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Al-Khazrajy, O.S.A.; Abdallh, M.; Alanazi, S.A. Modifications of polymers through the addition of ultraviolet absorbers to reduce the aging effect of accelerated and natural irradiation. Polymers 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; pp. 1–384. [Google Scholar]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]













| Complex | Color | M.P. (°C) | Yield (%) | Element Analysis (Calculated; %) | ||
|---|---|---|---|---|---|---|
| C | H | M | ||||
| Mn | Dark brown | 173–175 | 88 | 62.36 (62.27) | 7.58 (7.64) | 11.15 (10.95) |
| Co | Brown | 151–153 | 90 | 61.65 (61.78) | 7.42 (7.58) | 11.53 (11.66) |
| Ni | Light green | 300 (decomp) | 91 | 61.98 (61.78) | 7.50 (7.58) | 11.49 (11.62) |
| Metal Complex | FTIR, Frequency (ν, cm−1) | ||||
|---|---|---|---|---|---|
| Carboxylate Group (COO−) | C=C | M–O | |||
| asym | sym | Δν (asym − sym) | |||
| Ibuprofen sodium salt | 1697 | 1411 | 286 | 1551 | — |
| Mn | 1799 | 1408 | 391 | 1558 | 416 |
| Co | 1789 | 1400 | 389 | 1593 | 428 |
| Ni | 1797 | 1400 | 397 | 1589 | 425 |
| Complex | λ (nm) | Absorption (cm−1) | Transition | Λm (µS/cm) | μeff | Geometry | Hybridization |
|---|---|---|---|---|---|---|---|
| Ibuprofen sodium salt | 294 | 34,014 | π → π٭ | — | — | — | — |
| 298 | 33,557 | π → π٭ | |||||
| Ibuprofen-Mn | 300 | 33,333 | π → π٭ | 10 | 5.9 | Octahedral | sp3d2 high spin |
| 315 | 31,746 | π → π٭ | |||||
| 378 | 26,455 | 6A1g → 4T2g (D) | |||||
| 523 | 19,120 | 6A1g → 4T2g (G) | |||||
| 642 | 15,573 | 6A1g → 4T1g | |||||
| Ibuprofen-Co | 295 | 33,898 | π → π٭ | 10 | 4.5 | Octahedral | sp3d2 high spin |
| 304 | 32,895 | π → π٭ | |||||
| 343 | 29,155 | 4T1g(F) → 4A2g(F) | |||||
| 562 | 17,794 | 4T1g(F) → 4T1g(P) | |||||
| Ibuprofen-Ni | 295 | 33,898 | π → π٭ | 0 | 3.1 | Octahedral | sp3d2 high spin |
| 303 | 33,003 | π → π٭ | |||||
| 339 | 29,499 | 3A2g (F) → 3T1g (P) | |||||
| 417 | 23,981 | 3A2g (F) → 3T1g (F) |
| PS Additive | Reduction in Rq (by Fold) | Reference |
| Ibuprofen–Mn complex | 13.2 | Current work |
| Biphenyl-3,3′,4,4′-tetraamine Schiff bases | 8.3 | [18] |
| Cephalexin Schiff bases | 27.1 | [19] |
| 1,2,3,4-Triazole-3-thiol Schiff bases | 3.3 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, D.S.; Mohammed, A.; Husain, A.A.; El-Hiti, G.A.; Kadhom, M.; Kariuki, B.M.; Yousif, E. Fabrication of Highly Photostable Polystyrene Films Embedded with Organometallic Complexes. Polymers 2022, 14, 1024. https://doi.org/10.3390/polym14051024
Ahmed DS, Mohammed A, Husain AA, El-Hiti GA, Kadhom M, Kariuki BM, Yousif E. Fabrication of Highly Photostable Polystyrene Films Embedded with Organometallic Complexes. Polymers. 2022; 14(5):1024. https://doi.org/10.3390/polym14051024
Chicago/Turabian StyleAhmed, Dina S., Alaa Mohammed, Amani A. Husain, Gamal A. El-Hiti, Mohammed Kadhom, Benson M. Kariuki, and Emad Yousif. 2022. "Fabrication of Highly Photostable Polystyrene Films Embedded with Organometallic Complexes" Polymers 14, no. 5: 1024. https://doi.org/10.3390/polym14051024