The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PHBV Films
2.2. Analysis of Biodegradability under Thermophilic Composting
2.3. Analysis of Biodegradability under Vermicomposting (Mesophilic Condition)
2.4. Analysis of Biodegradability in Freshwater Biotype
2.5. Calculation of the Percentage Biodegradation
2.6. Calculation of Degree of Disintegration
2.7. Differential Scanning Calorimetry (DSC)
2.8. Thermogravimetric Analysis (TGA)
2.9. Scanning Electron Microscopy (SEM) Images
3. Results and Discussion
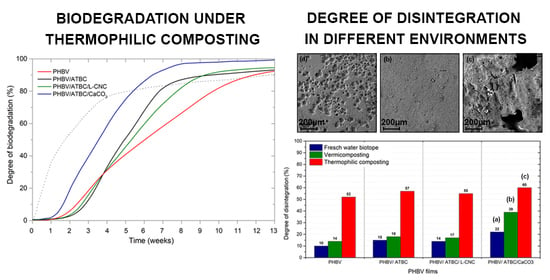
3.1. Determination of Ultimate Aerobic Biodegradability
3.2. Degree of Disintegration
3.3. Differential Scanning Calorimetry (DSC)
3.4. Thermogravimetric Analysis (TGA)
3.5. Scanning Electron Microscopy (SEM) Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Delaimy, W.; Ramanathan, V.; Sánchez Sorondo, M. Health of People, Health of Planet and Our Responsibility: Climate Change, Air Pollution and Health; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A. Predicted Growth in Plastic Waste Exceeds Efforts to Mitigate Plastic Pollution. J. Sci. 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Singh, O. Global Trends of Fossil Fuel Reserves and Climate Change in the 21st Century; InTech: Rijeka, Croatioa, 2012; Chapter 8; Volume 8. [Google Scholar]
- Puntes, V. Plastic-the Facts 2020. [Online]. B.m.: Plastic Europe Association of Plastic Manufactures. 2020. Available online: https://www.plasticseurope.org/en/resources/publications (accessed on 10 January 2022).
- D’ambrières, W. Plastics Recycling Worldwide: Current Overview and Desirable Changes. Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- Calabrò, P.S.; Grosso, M. Bioplastics and Waste Management. Waste Manag. 2018, 78, 800–801. [Google Scholar] [CrossRef]
- Shen, L.; Haufe, J.; Patel, M.K. Product Overview and Market Projection of Emerging Bio-Based Plastics. Utrecht Univ. Comm. Eur. Polysacch. Netw. Excell. Eur. Bioplast. 2009, 243, 1–245. [Google Scholar]
- Guo, M.; Stuckey, D.C.; Murphy, R.J. Is It Possible to Develop Biopolymer Production Systems Independent of Fossil Fuels? Case Study in Energy Profiling of Polyhydroxybutyrate-Valerate (PHBV). Green Chem. 2013, 15, 706–717. [Google Scholar] [CrossRef]
- Salomez, M.; George, M.; Fabre, P.; Touchaleaume, F.; Cesar, G.; Lajarrige, A.; Gastaldi, E. A Comparative Study of Degradation Mechanisms of PHBV and PBSA under Laboratory-Scale Composting Conditions. Polym. Degrad. Stab. 2019, 167, 102–113. [Google Scholar] [CrossRef]
- Conn, R.E.; Kolstad, J.J.; Borzelleca, J.F.; Dixler, D.S.; Filer, L.J., Jr.; LaDu, B.N., Jr.; Pariza, M.W. Safety Assessment of Polylactide (PLA) for Use as a Food-Contact Polymer. Food Chemi. Toxicol. 1995, 33, 273–283. [Google Scholar] [CrossRef]
- Jost, V. Packaging Related Properties of Commercially Available Biopolymers–An Overview of the Status Quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Grujić, R.; Vujadinović, D.; Savanović, D. Biopolymers as Food Packaging Materials. In Advances in Applications of Industrial Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 139–160. [Google Scholar]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Biopolymers for Food Packaging Applications. In Smart Polymers and their Applications; Woodhead Publishing: Cambridge, UK, 2014; pp. 476–509. [Google Scholar]
- Steinbüchel, A.; Lütke-Eversloh, T. Metabolic Engineering and Pathway Construction for Biotechnological Production of Relevant Polyhydroxyalkanoates in Microorganisms. Biochem. Eng. J. 2003, 16, 81–96. [Google Scholar] [CrossRef]
- Karak, N. Vegetable Oil-Based Polymers: Properties, Processing and Applications; Woodhead Publishing Limited: New Delhi, India, 2012; Chapter 2; Volume 1. [Google Scholar]
- Aitor, L.; Erlantz, L. A Review on the Thermomechanical Properties and Biodegradation Behaviour of Polyester. Eur. Polym. J. 2019, 121, 1–31. [Google Scholar]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of Recent Advances in the Biodegradability of Polyhydroxyalkanoate (PHA) Bioplastics and Their Composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. A Study of Physical and Mechanical Properties of Poly (Hydroxybutyrate-Co-Hydroxyvalerate) during Composting. Polym. Degradat. Stab. 2003, 80, 59–66. [Google Scholar] [CrossRef]
- Rutkowska, M.; Krasowska, K.; Heimowska, A.; Adamus, G.; Sobota, M.; Musioł, M.; Janeczek, H.; Sikorska, W.; Krzan, A.; Žagar, E. Environmental Degradation of Blends of Atactic Poly [(R, S)-3-Hydroxybutyrate] with Natural PHBV in Baltic Sea Water and Compost with Activated Sludge. J. Polym. Environ. 2008, 16, 183–191. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Ssci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, X.-L.; Wang, Y.-Z. Biodegradation Behavior of PHAs with Different Chemical Structures under Controlled Composting Conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Renard, E.; Walls, M.; Guérin, P.; Langlois, V. Hydrolytic Degradation of Blends of Polyhydroxyalkanoates and Functionalized Polyhydroxyalkanoates. Polym. Degradat. Stab. 2004, 85, 779–787. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A Model for Hydrolytic Degradation and Erosion of Biodegradable Polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Herzog, K.; Müller, R.-J.; Deckwer, W.-D. Mechanism and Kinetics of the Enzymatic Hydrolysis of Polyester Nanoparticles by Lipases. Polym. Degradat. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Buchholz, V.; Agarwal, S.; Greiner, A. Synthesis and Enzymatic Degradation of Soft Aliphatic Polyesters. Macromol. Biosci. 2016, 16, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J.; Kersters, K. Biodegradation of Polyhydroxyalkanoates. FEMS Microbiol. Rev. 1992, 9, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.-X.; Wang, Y.; Wang, X.-L.; Wang, Y.-Z. Biodegradation Behavior of PHBV Films in a Pilot-Scale Composting Condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Dorgan, J.R.; Schilling, K. Water Transport in Polylactic Acid (PLA), PLA/Polycaprolactone Copolymers, and PLA/Polyethylene Glycol Blends. J. Environ. Polym. Degrad. 1997, 5, 125–136. [Google Scholar]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of Poly (Lactic Acid)(PLA), Poly (3-Hydroxybutyrate-Co-Valerate)(PHBV) and PLA/PHBV Blend in Compost and Soil Environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Chen, H. Assessment of Biodegradation in Different Environmental Compartments of Blends and Composites Based on Microbial Poly (Hydroxyalkanoate)s. Pisa Univ. Pisa 2012, ill, 1–191. [Google Scholar]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A Processing, Characterization and Marine Biodegradation Study of Melt-Extruded Polyhydroxyalkanoate (PHA) Films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Deroiné, M.; César, G.; Le Duigou, A.; Davies, P.; Bruzaud, S. Natural Degradation and Biodegradation of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) in Liquid and Solid Marine Environments. J. Polym. Environ. 2015, 23, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic Anaerobic Biodegradation Test and Analysis of Eubacteria Involved in Anaerobic Biodegradation of Four Specified Biodegradable Polyesters. Polym. Degrad. Stab. 2013, 98, 1182–1187. [Google Scholar] [CrossRef]
- Boonmee, J.; Kositanont, C.; Leejarkpa, T. Biodegradation of Poly (Lactic Acid), Poly (Hydroxybutyrate-Co-Hydroxyvalerate), Poly (Butylene Succinate) and Poly (Butylene Adipate-Co-Terephthalate) under Anaerobic and Oxygen Limited Thermophilic Conditions. Environ. Asia 2016, 9, 107–115. [Google Scholar]
- Lammi, S.; Gastaldi, E.; Gaubiac, F.; Angellier-Coussy, H. How Olive Pomace Can Be Valorized as Fillers to Tune the Biodegradation of PHBV Based Composites. Polym. Degrad. Stab. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- David, G.; Michel, J.; Gastaldi, E.; Gontard, N.; Angellier-Coussy, H. How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites. Int. J. Mol. Sci. 2020, 21, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meereboer, K.W.; Pal, A.K.; Cisneros-López, E.O.; Misra, M.; Mohanty, A.K. The Effect of Natural Fillers on the Marine Biodegradation Behaviour of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate)(PHBV). Sci. Rep. 2021, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal Mechanical Properties of Biodegradable Natural Rubber-Toughened PHBV Bioplastics Intended for Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly (3-Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylene Succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.-G. Plasticizing Polylactide—The Effect of Different Plasticizers on the Mechanical Properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The Plasticizer Market: An Assessment of Traditional Plasticizers and Research Trends to Meet New Challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Jost, V.; Langowski, H.-C. Effect of Different Plasticisers on the Mechanical and Barrier Properties of Extruded Cast PHBV Films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Chen, X.; Pan, J.; Xu, K. Miscibility, Crystallization Kinetics, and Mechanical Properties of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV)/Poly (3-hydroxybutyrate-co-4-hydroxybutyrate)(P3/4HB) Blends. J. Appl. Polym. Sci. 2010, 117, 838–848. [Google Scholar] [CrossRef]
- Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. Biodegradation of Poly (Lactic Acid) Biocomposites under Controlled Composting Conditions and Freshwater Biotope. J. Polym. 2021, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Husarova, L.; Machovsky, M.; Gerych, P.; Houser, J.; Koutny, M. Aerobic Biodegradation of Calcium Carbonate Filled Polyethylene Film Containing Pro-Oxidant Additives. Polym. Degrad. Stab. 2010, 95, 1794–1799. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Heidarian, P.; Behzad, T.; Sadeghi, M.; Bagheri, R. Effect of Calcium Carbonate Nanoparticles on Barrier Properties and Biodegradability of Polylactic Acid. Fibers Polym. 2017, 18, 2041–2048. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.N.; Jafarzadeh, S.; Paridah, M.T.; Gopakumar, D.A.; Tajarudin, H.A.; Thomas, S.; Abdul Khalil, H.P.S. Enhancement in the Physico-Mechanical Functions of Seaweed Biopolymer Film via Embedding Fillers for Plasticulture Application—A Comparison with Conventional Biodegradable Mulch Film. J. Polym. 2019, 11, 210. [Google Scholar]
- Suharty, N.S.; Almanar, I.P.; Dihardjo, K.; Astasari, N. Flammability, Biodegradability and Mechanical Properties of Bio-Composites Waste Polypropylene/Kenaf Fiber Containing Nano CaCO3 with Diammonium Phosphate. Procedia Chem. 2012, 4, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Nekhamanurak, Y.B.; Patanathabutr, P.; Hongsriphan, N. Mechanical Properties of Hydrophilicity Modified CaCO3-Poly (Lactic Acid) Nanocomposite. J. Appl. Phys. Math. 2012, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- Nekhamanurak, B.; Patanathabutr, P.; Hongsriphan, N. The Influence of Micro-/Nano-CaCO3 on Thermal Stability and Melt Rheology Behavior of Poly (Lactic Acid). Energy Procedia 2014, 56, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of Lignin in a Compost Environment: A Review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Micales, J.A.; Skog, K.E. The Decomposition of Forest Products in Landfills. Int. Biodeterior. Biodegrad. 1997, 39, 145–158. [Google Scholar] [CrossRef]
- Volova, T.G.; Gladyshev, M.I.; Trusova, M.Y.; Zhila, N.O. Degradation of Polyhydroxyalkanoates in Eutrophic Reservoir. Polym. Degrad. Stab. 2007, 92, 580–586. [Google Scholar] [CrossRef]
- Tsou, C.-H.; Suen, M.-C.; Yao, W.-H.; Yeh, J.-T.; Wu, C.-S.; Tsou, C.-Y.; Chiu, S.-H.; Chen, J.-C.; Wang, R.Y.; Lin, S.-M. Preparation and Characterization of Bioplastic-Based Green Renewable Composites from Tapioca with Acetyl Tributyl Citrate as a Plasticizer. Materials 2014, 7, 5617–5632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased Additive Plasticizing Polylactic Acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Guinault, A.; Avérous, L.; Ducruet, V. Analysis of the Structure-Properties Relationships of Different Multiphase Systems Based on Plasticized Poly (Lactic Acid). J. Polym. Environ. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Martino, L.; Berthet, M.-A.; Angellier-Coussy, H.; Gontard, N. Understanding External Plasticization of Melt Extruded PHBV–Wheat Straw Fibers Biodegradable Composites for Food Packaging. J. Appl. Polym. Sci. 2015, 132, 41611. [Google Scholar] [CrossRef]
- Kirboga, S.; Öner, M. Oxygen Barrier and Thermomechanical Properties of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biocomposites Reinforced with Calcium Carbonate Particles. Acta Chim. Slov. 2020, 67, 137–150. [Google Scholar] [CrossRef]
- Kirboga, S.; Öner, M. The Properties of Phbv/CaCO3 Composites Prepared By Melt Processing. In 6 th Icntc Book of Abstracts; ICNTC Secretariat: Istambul, Turkey, 2020; p. 102. [Google Scholar]
- Wang, K.Y.; Cao, F. Effect of CaCO3 on Thermal and Crystalline Morphology Properties of Biodegradable PHBV. In Proceedings of the Advanced Materials Research. Trans. Tech. Publ. 2013, 781, 542–545. [Google Scholar]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Mintz, E.A. Lignin-Coated Cellulose Nanocrystals as Promising Nucleating Agent for Poly (Lactic Acid). J. Therm. Anal. Calorim. 2016, 126, 1243–1251. [Google Scholar] [CrossRef]
- Borůvka, M.; Běhálek, L.; Novák, J. Properties and Crystallization of PLLA Biopolymers with Cellulose Nanocrystals and Organic Plasticizer. MM Sci. J. 2020, 2020, 4080–4085. [Google Scholar] [CrossRef]
- Liu, T.; Petermann, J. Multiple Melting Behavior in Isothermally Cold-Crystallized Isotactic Polystyrene. Polymer 2001, 42, 6453–6461. [Google Scholar] [CrossRef]
- Gunaratne, L.; Shanks, R.A. Multiple Melting Behaviour of Poly (3-Hydroxybutyrate-Co-Hydroxyvalerate) Using Step-Scan DSC. Eur. Polym. J. 2005, 41, 2980–2988. [Google Scholar] [CrossRef]
- Erceg, M.; KovaČiĆ, T.; KlariĆ, I. Thermal Degradation of Poly (3-Hydroxybutyrate) Plasticized with Acetyl Tributyl Citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Fang, X.; Liao, J.; Zhou, X.; Zhou, S.; Bai, F.; Peng, S. High Solid Content Production of Environmentally Benign Ultra-Thin Lignin-Based Polyurethane Films: Plasticization and Degradation. Polymer 2019, 178, 121572. [Google Scholar] [CrossRef]







| Sample Designation | Composition (wt. %) | |||
|---|---|---|---|---|
| PHBV | ATBC | L-CNC | CaCO3 | |
| PHBV | 100 | - | - | - |
| PHBV/ATBC | 90 | 10 | - | - |
| PHBV/ATBC/L-CNC | 89 | 10 | 1 | - |
| PHBV/ATBC/CaCO3 | 80 | 10 | - | 10 |
| Sample Designation | Proportions (%) |
|---|---|
| PHBV | 55.8 |
| ATBC | 59.7 |
| L-CNC | 44.4 |
| CaCO3 | - |
| Sample Designation | PLA | PLA/ATBC | PLA/ATBC/ L-CNC | PLA/ATBC/ CaCO3 | PBHV | PHBV/ ATBC | PHBV/ATBC/L-CNC | PHBV/ATBC/CaCO3 |
|---|---|---|---|---|---|---|---|---|
| 30 days | 6% | 16% | 15% | 58% | 32% | 35% | 33% | 56% |
| 45 days | 10% | 31% | 27% | 83% | 54% | 73% | 62% | 88% |
| 60 days | 17% | 69% | 60% | 94% | 72% | 88% | 85% | 98% |
| 75 days | 42% | 97% | 90% | 100% | 87% | 92% | 93% | 99% |
| Sample Designation | Exposition Time (Months) | Tcc (°C) | ∆Hcc (J/g) | Tm1 (°C) | Tm2 (°C) | ∆Hm (J/g) | XC (%) |
|---|---|---|---|---|---|---|---|
| PHBV | Initial state | 121.8 | 84.2 | 169.6 | 173.3 | 82.2 | 56 |
| Vermicompost | 122.2 | 81.7 | 167.3 | 171.5 | 77.7 | 53 | |
| Freshwater biotope | 122.3 | 84.0 | 168.9 | 174.2 | 83.6 | 57 | |
| Thermophilic composting | 121.4 | 82.9 | 167.0 | 173.1 | 76.2 | 52 | |
| PHBV/ATBC | Initial state | 119.7 | 81.2 | 166.4 | 170.1 | 80.5 | 61 |
| Vermicompost | 119.6 | 80.4 | 169.2 | 79.2 | 60 | ||
| Freshwater biotope | 118.5 | 73.8 | 162.7 | 169.9 | 72.6 | 55 | |
| Thermophilic composting | 119.6 | 78.3 | 165.3 | 170.5 | 73.9 | 56 | |
| PHBV/ATBC/L-CNC | Initial state | 119.8 | 74.3 | 150.8 | 170.6 | 70.1 | 54 |
| Vermicompost | 118.5 | 76.2 | 172.4 | 77.0 | 59 | ||
| Freshwater biotope | 120.3 | 74.7 | 160.9 | 171.0 | 71.2 | 55 | |
| Thermophilic composting | 119.0 | 75.1 | 161.3 | 170.2 | 73.1 | 56 | |
| PHBV/ATBC/CaCO3 | Initial state | 119.1 | 65.5 | 160.6 | 169.5 | 62.2 | 53 |
| Vermicompost | 118.8 | 69.8 | 170.8 | 68.0 | 58 | ||
| Freshwater biotope | 119.9 | 64.5 | 161.2 | 170.3 | 59.8 | 51 | |
| Thermophilic composting | 118.3 | 67.6 | 162.2 | 169.9 | 63.3 | 54 | |
| Sample Designation | Exposition Time (Months) | |||||||
|---|---|---|---|---|---|---|---|---|
| Initial State | Vermicomposting | Freshwater Biotope | Thermophilic Composting | |||||
| T3 (%) | T50 (%) | T3 (%) | T50 (%) | T3 (%) | T50 (%) | T3 (%) | T50 (%) | |
| PHBV | 270.9 | 290.0 | 260.1 | 276.8 | 271.8 | 289.4 | 243.8 | 265.5 |
| PHBV/ATBC | 253.2 | 286.3 | 249.8 | 281.6 | 251.4 | 271.0 | 237.2 | 261.7 |
| PHBV/ATBC/ L-CNC | 254.6 | 287.1 | 251.1 | 284.8 | 257.7 | 284.9 | 244.3 | 275.8 |
| PHBV/ATBC/ CaCO3 | 237.3 | 267.6 | 226.3 | 258.5 | 229.9 | 245.9 | 217.3 | 267.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites. Polymers 2022, 14, 838. https://doi.org/10.3390/polym14040838
Brdlík P, Borůvka M, Běhálek L, Lenfeld P. The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites. Polymers. 2022; 14(4):838. https://doi.org/10.3390/polym14040838
Chicago/Turabian StyleBrdlík, Pavel, Martin Borůvka, Luboš Běhálek, and Petr Lenfeld. 2022. "The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites" Polymers 14, no. 4: 838. https://doi.org/10.3390/polym14040838